Operative Laparoscopy
Authors
INTRODUCTION
Endoscopic surgery is believed to have had its origin in the Babylonian Talmud (Table 1). This description of a lead funnel with a bent mouthpiece and a wooden drainpipe used for visual inspection of the vagina and cervix set the stage for development of a surgical tool that continues to have ever-increasing application in the current surgical armamentarium. Guilio Cesare Aranzi has been credited with the discovery of the endoscopic light source in 1587. His camera obscura was invented by the monk Don Panuce. Further expertise was contributed by Arnaud, who in 1798 reported on a dark lantern for medical endoscopic examination.
From the current perspective of modern endoscopy, among its earliest foundations may have been Bozzini's invention in 1805 of the light reflector. After this landmark came the culmination of refinement in endoscopic surgery of that era, when Desormeaux in 1853 developed the first practical endoscope.
Photographic and video imaging, as it is currently known, was first described in 1874 by Stein in Frankfurt, Germany. His photoendoscope permitted researchers such as Pantaleoni (1868) to construct what is now termed the hysteroscope. A cool light source for cystoscopic assessment was developed in 1879 by Nitze.
In the 1900s, one of the first reports on operative laparoscopy came from Boesch in Switzerland, who published work on laparoscopic evaluation of female pelvic organs. In 1955, Fikentscher and Semm described a universal insufflation system.
Palmer described a celioscopic technique for infertility evaluation. Carbon dioxide pneumoperitoneum was first described by Semm in 1967. Rapidly after that was the development of endocoagulation techniques to accomplish hemostasis (Semm 1973). The availability of the pediatric-size Hopkins rod lens telescopes was a contribution from Gans (Germany). His initiative led to the Storz research and development department, in essence, designing the first laparoscope for use in the pediatric patient. Innovative thinking, rapid technologic advancement, and evidence for the overall efficacy of minimally invasive surgery have been the sequela to the historical foundation provided by these pioneers in gynecology and general surgery.
In the early 1990s, microlaparoscopy (smaller than 2-mm trocars) emerged as a powerful technology that brought diagnostic and operative laparoscopy into the office setting using minimal anesthesia.
In 1993, the development of an ultrasonically activated scalpel (Harmonic scalpel; Ethicon, Cincinnati, OH) for laparoscopic use was described. This initiated a drive toward an ideal mechanical energy form for laparoscopy with optimal safety for the patients.
Robotic technology was introduced to laparoscopic surgery in 1994. A robotic assistant, the voice-controlled optic holder, AESOP (Automated Endoscopic System for Optimal Positioning), was used to hold and maneuver the laparoscopic camera. Although cost-prohibitive and utilized in specific locations wordwide, telerobotics-assisted laparoscopic surgery is a technique in which an experienced surgeon can control the laparoscopic camera and mentor an inexperienced surgeon. Since the introduction of the da Vinci Surgical System (Intuitive Surgical), this device has become the most widely utilized system in the United States. A full cadre of surgeons from various disciplines have begun evaluations for specific procedures. For gynecology, the da Vinci may facilitate procedures that require extensive suturing, such as myomectomy or sacrocolpopexy. Although costly and relatively new to our field, studies are underway which may further validate the da Vinci as a tool for our surgical armamentarium.
Table 1. History of endoscopy surgery
| Date | Source | Contribution |
| Ancient Greece | Babylonian Talmud | Mouthpiece drain pipe to inspect vagina and cervix |
| 1587 | Guilio C. Arnazi | Camera obscura |
| 1798 | Arnaud | Dark lantern for endoscopic examination |
| 1874 | Stein | Photographic and video imaging |
| 1935 | Boesch | First laparoscopic evaluation of female patient |
| 1955 | Fikenstscher | Insufflation apparatus |
| 1961 | USA | The Unimate, the first industrial robot is built for use in the automotive industry |
| 1967 | Semm | CO2 pneumoperitoneum |
| 1973 | Semm | Endocoagulation for hemostasis |
| 1991 | USA | Microlaparoscopy (<2-mm trocar) |
| 1993 | Ethicon (Cincinnati, OH) | Ultrasonic Energy (harmonic scalpel) |
| 1994 | Computer Motion (California) | AESOP robotic visualization |
| 1996 | International contribution | Six million components are placed on a single chip, increasing the power of the integrated circuit |
| 1997 | USA | The Sojourner, the first robotic rover in space, roams on Mars |
| 1998 | Computer Motion | HERMES (Computer Motion, control center robotics) |
| 1999 | Computer Motion | ZEUS (Computer Motion) robotic surgical system introduced |
| 2000 | Karl Storz (Germany) | Endobag extractor |
| Ethicon | Facilitates laparoscopic intact mass removal with Endobag |
INSTRUMENTATION
Endoscopy is possible in infants and children as well as in adolescents and adults. With the advent of 1.2–3-mm laparoscopes, new applications for endoscopic surgery have resulted. In the infant or pediatric patient, laparoscopes ranging from 1.2 to 10 mm in diameter can be used with 0- or 30-degree lens configurations. The former provides a head-on view; the latter is indicated for endoscopic suturing or when a structure must be assessed at an angle and placement of a second port is not deemed necessary. Most gynecologic surgeons are accustomed to the 0-degree lens since most pelvic organs are relatively midline. The 30 degree does offer some advantages, however, in that by rotating the lens, access to laterally deviated organs or structures may be facilitated. Laparoscopic equipment has become progressively lighter in weight. Resolution has significantly improved with both three-chip technology and now high-definition lenses and monitors. The number of pixels associated with the video equipment is an important parameter to consider when selecting the type of video for the operating room. Laparoscopic surgical instrument companies have designed single-piece digital laparoscopes that obviate the need for traditional camera heads.
Laparoscopic trocars come in sizes of 1.2–15 mm; instrumentation is designed to utilize both smaller and larger trocars. The SonoSurg trocar by Olympus uses the harmonic scalpel with a specially designed tip that provides the energy for penetrating the anterior rectus fascia (lateral ports only) on insertion. This instrument not only provides a means of entry that requires less force, but may be a cost-effective tool when using harmonics during the operative procedure. US Surgical has designed several trocar types, but one of interest is the radially expanding VersaStep trocar, which comes in both 5 and 11 mm. This results in stretching the fascial fibers as opposed to tearing them, thereby decreasing the facial defect. Most corporations vested in trocar development have designed systems based on bladeless technology that split rather than cut fascial fibers. Examples include the radially expanding VersaStep by US Surgical (single-puncture technique is used with a Veress-type needle to obtain pneumoperitoneum, after which a blunt conical tip is passed through an overlying mesh sheath) and the Endopath XCEL by Ethicon Endosurgical (functions similar to driving a screw with a screwdriver and will accept a lens to actively view peritoneal entry) (Fig. 1). Implied in their use is safety, smaller fascial defects and less risk of postoperative herniation. None of these have truly been validated because of the low prevalence of such catastrophic events (vascular or visceral penetration), and most are comparable in cost. Several manufacturers still make bladed trocars for those accustomed to their use.
Fig. 1. Xcel trocar (Ethicon, Cincinnati, OH).
Ultrasonically activated devices (Harmonic scalpel) were developed to provide dissecting and coagulating capabilities without the use of electrical current. The advantage of this form of energy is that it is quite versatile and may be used to dissect, incise, desiccate, and coagulate. Thermal injury is a possible hazard as with any energy source (kinetic energy); however, the risk of electrical capacitance is eliminated and thermal spread is typically less than with electrosurgical instrumentation. Many gynecologic and general surgeons alike prefer this modality, which is frequently used for excision of endometriosis, myomectomy, total laparoscopic hysterectomy (TLH), ectopic gestation, and adhesiolysis. Vessel sealing capacity is the primary drawback, as the upper limit of vessel size is approximately 3 mm. The newest version to be released is the Ethicon ACE.
Newer devices that use bipolar energy have been developed to facilitate advanced endoscopic procedures. Two such devices include those produced by Gyrus/ACMI and Covidian LigaSure division. Each manufactures vessel sealing and dividing forceps (Cutting Forceps and Trissector from Gyrus/ACMI; LigaSure V and Atlas by Covidian) as well as those which simply coagulate and dissect (Lyons Dissector from Gyrus/ACMI; LigaSure Lap by Covidian). Price, efficacy of seal, and thermal spread are relatively comparable. The Enseal device by Surgrx is another vessel sealing instrument that is quite similar in form, function, and cost. The 5 mm Lyons dissector is well-adapted for excising endometriosis and working in tight spaces. The LigaSure device comes in both a 5 and 10 mm version that is a tripolar type instrument that seals and transects larger vascular pedicles with limited thermal spread.
When using monopolar energy, most experts recommend using disposable devices in order to limit the risk of inadvertent thermal spread from breaks in the insulation that occur with frequent use. Although Valleylab utilizes a system to minimize the risk of thermal accidents, Encision is the only patented system to eliminate capacitance and insulation breach. A second monitor is coupled to the primary electrosurgical generator to perform this function, in concert with the instrument that utilizes a reusable plastic grasper with a disposable electrode and outer insulator. Although non-visualized thermal injuries are rare, this technology may afford extra insurance for those who frequently use this form of energy.
With improved lens clarity, microlaparoscopy now provides sufficient visualization and can be used in a variety of situations. Because of cost implications, office laparoscopy and pain mapping under moderate sedation are two such procedures that have potential in the managed care arena. Microlaparoscopic tubal ligation under local anesthesia has also been well-described in the literature.1, 2, 3, 4
Specimen collection can be successfully accomplished with various laparoscopically placed devices (Ethicon Endobag, Karl Storz Endobag extractor). These serve to remove masses during laparoscopy and were introduced in 1999. The Endobag extractor has three curved and removable blades in two different sizes, 5 and 7 cm. The grip of the extractor can be clicked into the three blades, forming a speculum-like instrument. This new instrument allows removal of surgical specimens for any type of Endobag without the risk of mass rupture. This instrument can revolutionize the laparoscopic role in the management of gynecologic malignancy.5 Larger masses may require the use of a 15-mm port.
ANESTHESIA
The surgeon must understand several anesthesia-related concerns. The first is proper positioning of the patient. She must be placed in the lithotomy position, ideally, standing in stirrups. Care must be taken to avoid pressure on the popliteal fossa. Effort must be made to prevent femoral neuropathy, which has been associated with laparoscopic procedures.6, 7 The patient probably will be in steep Trendelenburg position. Decreased vital capacity and functional residual capacity (18% and 14.5%, respectively) result from compromised diaphragmatic excursion and increased pulmonary blood volume.8 Pneumoperitoneum established with carbon dioxide (or nitrous oxide) at abdominal pressures of 12–16 mmHg is associated with respiratory and metabolic acidosis. The decrease in pH occurs simultaneously with a decrease in arterial oxygen tension. The surgeon should be cognizant of cardiac arrhythmia, especially the more common ones, and should be knowledgeable regarding treatment modalities. Hypoxia and vasovagal reflexes from peritoneal stimulation and distention, along with light anesthesia, can result in arrhythmia.
A list of premedications for adults undergoing gynecologic endoscopy is shown in Table 2. The surgeon must be well-versed in the medications used in the induction of anesthesia (Table 3) and must understand the types of maintenance anesthesia commonly used for endoscopic surgery (Table 4). Basic knowledge of anesthesia as germane to the laparoscopic procedure will help avert complications. The anesthesiologist should be requested to avoid the use of nitrous oxide gas, because its use has been associated with obscuring loops of distended bowel, although this may be more anecdotal than scientific.
Table 2. Premedication for adults undergoing gynecologic endoscopy*
| Drug | Dosage (mg) | Route of Administration |
| ANTICHOLINERGICS | ||
| Atropine sulfate | 0.4–0.8 | IM or IV |
| Scopolamine | 0.4–0.6 | IM or IV |
| Glycopyrrolate | 0.2–0.4 | IM or IV |
| GASTRIC ANTISECRETAGOGUES | ||
| Cimetidine (Tagamet) | 200–400 | PO, IM, or IV |
| Ranitidine (Zantac) | 50–100 | PO, IM, or IV |
| Famotadine | 10–20 PO | IV |
| ANTIEMETICS | ||
| Droperidol (Inapsine) | 0.0625–0.1250 | IM or IV |
| Promethazine (Phenergan) | 77.5–25.0 | PO, IM, or IV |
| Metoclopramide (Reglan) | 10–20 | PO, IM, or IV |
| SEDATIVES AN ANXIOLYTICS | ||
| Diazepan (Valium) | 5–20 | IV |
| Hydroxyzine (Vistaril) | 25–100 | IM |
| Midazolam (Versed) | 205–5.0 | IM or IV |
*Typical 70-kg ASS class I or II patients; geriatric patients require a dose reduction up to one-third of the above-mentioned dosages.
(Lucas LF, Rigor BM: Anesthesia. In Sanfilippo JS, Levine RL, [eds]: Operative Gynecologic Endoscopy, p 387. 2nd ed. New York, Springer Verlag, 1996.)
Table 3. Induction of anesthesia
| Drug | Effect |
| Barbiturates | |
| Thiopental | Prolonged recovery |
| Methohexital | Hiccoughs, myoclonus |
| Nonbarbiturates | |
| Propofol (Diprivan) | Antiemetic, rapid recovery |
| Etomidate | Nausea and vomiting, involuntary motor activity |
| Ketamine (Ketalar) | Dissociative anesthetic, psychomimetic effects |
(Lucas LF, Rigor BM: Anesthesia. In Sanfilippo JS, Levine RL, [eds]: Operative Gynecologic Endoscopy, p. 368. 2nd ed. New York, Springer Verlag, 1996).
Table 4. Maintenance of anesthesia
| Generic Name | Trade Name | Comments |
| INHALATION AGENTS | ||
| Fluothane | Halothane | Cardiac arrhythmia |
| Enflurane | Ethrane | Rapid recovery, fewer side effects in outpatients, potent respiratory and CNS depressant |
| Isoflurane | Forane increased nausea/dizziness in outpatients | |
| Desflurane | Suprane | |
| INTRAVENOUS AGENTS | ||
| Fentanyl | Sublimaze | |
| Sufentanil | Sufenta | |
| Alfentanil | Alfenta |
(Lucas LF, Rigor BM: Anesthesia. In Sanfilippo JS, Levine RL, [eds]: Operative Gynecologic Endoscopy, p 370. 2nd ed. New York, Springer Verlag, 1996)
ANTIBIOTIC PROPHYLAXIS
The use of preoperative antibiotics has been clearly shown to decrease the infectious morbidity associated with major surgery. Multiple randomized studies have demonstrated efficacy of antibiotics to reduce postoperative infections in women undergoing hysterectomy.9 There are insufficient data to recommend antibiotics for laparoscopic procedures when the vagina or bowel is not entered. The American College of Obstetricians and Gynecologists recommend that major endoscopic procedures be regarded similarly to their laparotomy counterparts and that otherwise clean procedures do not require preoperative antibiotics.10
PREVENTION OF VENOUS THROMBOEMBOLISM
The morbidity and mortality related to venous thromboembolism is sufficiently high to recommend prophylaxis in certain patients undergoing pelvic surgery. The overall incidence of venous thromboembolism in untreated patients may be as high as 16% in the gynecologic population; however, with appropriate prophylaxis, the incidence decreases to approximately 7%. The incidence of pulmonary embolism is typically less than 1%.11 Catheline and associates evaluated more than 2300 laparoscopic cases in which all patients received low-molecular-weight heparin and noted an incidence of 0.33%. No pulmonary embolisms were documented and all events occurred in patients at moderate risk. Risk factors included age older than 40, obesity, and long-standing pneumoperitoneum without release during the procedure. They concluded that thromboprophylaxis should be administered to patients undergoing laparoscopic procedures similarly to that administered to patients for laparotomy.12 Although the risks may be lessened with decreased hospital stay and early ambulation associated with endoscopic surgery, the procedures often require longer operating times while in Trendelenburg position. A recent publication which analyzed data from the University Healthsystem Consortium Clinical Database determined a lower risk of thromboembolic events in patients undergoing laparoscopic surgery compared to their laparotomic counterparts.13 Choices of agents include elastic stockings, sequential compression devices, and unfractionated and low-molecular-weight heparin, although current guidelines recommend compression stockings rather than elastic. A heparin compound is recommended for surgical patients at high risk (age older than 40 plus previous event, varicosities, infection, malignancy, estrogen therapy, obesity, prolonged surgery). Conversely, no treatment is required for patients at low risk (age younger than 40, surgery lasting less than 30 minutes).
TECHNIQUE
Until a few years ago, one of the first prerequisites for laparoscopy would have been general anesthesia, but office laparoscopy currently is performed with conscious sedation.14, 15 Generally, these procedures are diagnostic, and minimal operative procedures are performed; for more complex procedures, general anesthesia still is advisable. As the saying goes, preparation is everything, and laparoscopy is no exception. Before beginning the procedure, the bladder is emptied and, if necessary, the stomach is suctioned to prevent injury at the time of trocar placement. The patient is placed in the lithotomy position. In adults or adolescents, a cervical cannula is placed for uterine manipulation during the procedure. Several types of cannulas are available that allow for uterine manipulation and chromopertubation. Disposable catheters such as the HUMI are quite flexible and small in caliber, which are useful for younger or nulliparous patients. Reusable manipulators such as the Pelosi and Valtchev are heavier articulating tools that come with several adaptable head pieces and provide excellent laparoscopic exposure.
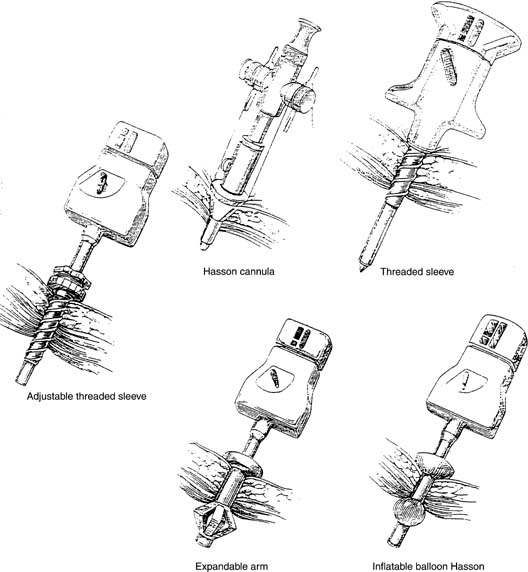
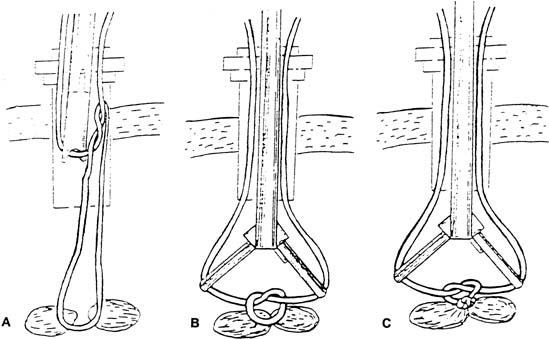
A small incision is made within the umbilicus and should be large enough to accommodate a 5- or 10–12-mm trocar. A Veress needle, directed at the inferior margin of the umbilicus (the aponeurosis), may be placed in the incision for insufflation. Obtaining a pneumoperitoneum with incorrect placement of the Veress needle has been associated with complications. Because most laparoscopic injuries occur on entry, aspirating with a 5-mL syringe, performing a water-drop test, and observing lower opening pressures (<8 mmHg) are essential before insufflating with carbon dioxide gas. Several studies have shown that direct trocar insertion can be equally effective, obtaining a more rapid pneumoperitoneum.16, 17 In a series of 1655 laparoscopic procedures, Hasaniya and colleagues18 report no complications from direct trocar insertion. In patients who have had previous abdominal surgery, in children, and in pregnant patients, an open technique may be preferred. Vascular and abnormally situated bowel injuries may be reduced with this technique because the fascia and peritoneum are incised under direct visualization;19 however, similar complication rates with both closed and open laparoscopy have been reported.20 Many surgeons use a Hasson cannula with a blunt trocar for open laparoscopy. With this technique, two sutures are placed through the fascia, lateral to the incision, and secured to a bar along the proximal shaft of the cannula to prevent loss of gas. Several new disposable cannulas are available with an inflatable balloon (Marlow, Willoughby, OH) or an expandable arm (Surgiport; US Surgical, Norwalk, CT), eliminating the need for stay sutures. Also, a cannula with a threaded sheath (Endopath, Somerville, NJ) is available that is literally screwed into the fascia to stabilize the sleeve (Fig. 2).21 The fascial defect must be reapproximated on completion of the procedure.
|
In 1999, the Food and Drug Administration (FDA) approved a new trocar system, InnerDyne. This system has a radially expanding access. It uses a 1.9-mm insufflation needle fitted into a radially expandable sleeve to penetrate the abdominal wall. The needle is withdrawn, leaving the expandable sleeve in place. The sleeve preserves the needle tract and becomes the access channel through which a blunt cannula or dilator is advanced, eliminating axial force during entry. The sleeve translates linear force to radial force.
Two other alternatives to open laparoscopy are the Visiport (US Surgical) and the Endopath Optiview (Ethicon), which permit visualization for cutting the subcuticular tissue and fascia (Fig. 3).
The Optiview allows entry into the abdominal cavity under direct vision. An incision can be made through the abdominal layers under direct vision. The Optiview allows identification of abdominal layers as they are dilated with the blunt conical tip. This method provides an easy way to avoid intestinal and vascular injury during initial trocar entry and permits access to the retroperitoneum for lymphadenectomy and incontinence procedures without entry into the peritoneal cavity.22 The latest version is called the Endopath XCEL, with the primary difference being the design of the diaphragm and trapping system that allows for easier removal of tissue without release of pneumoperitoneum.
If the surgeon prefers to insufflate the abdomen before trocar placement, insufflation needles 3.6 mm in diameter are available that have a transparent conical tip. A small laparoscope then can be attached to this needle for visualization during insertion.23
Many different sizes of trocars are available. Most laparoscopists preferred to place a 10–12-mm trocar at the umbilicus, as is necessary for a Hasson cannula; however, with improvements in camera and lens technology, modern 5 mm equipment is comparable to the former 10 mm. Left upper quadrant port placement may be used when peri-umbilical adhesions are suspected, or if the pathology is above the umbilicus. The stomach should be deflated with suction tubing and a 5-mm trocar directed to just beneath the twelfth rib in the midaxillary line. Accessory ports may then be placed in the lower abdomen, typically one in the midline and two lower quadrant ports. Care must be taken to avoid vessels when placing ports in the lower abdomen. The inferior epigastric vessels are subfacial and cannot be seen during transillumination since they are located posterior to the rectus sheath. They can, however, be seen from within the abdomen, lateral to the insertion of the round ligament. First, identify the insertion of the round ligament into the inguinal canal; then, follow the obliterated umbilical artery cephalad along the anterior abdominal wall. The vessels can often be seen in a ridge lateral to this embryologic remnant. Direct the trocar perpendicular to the abdominal wall and avoid wandering on entry. Transillumination of the superficial branch of the epigastric arteries, after pneumoperitoneum has been achieved, is helpful. The mean distance between these two vessels has been reported to be as far as 1.4 cm.24
Cannulas are constructed of stainless steel, plastic, or fiberglass. Most metal cannulas are insulated; nevertheless, a mixture of metal and plastic or fiberglass can create a capacitance effect (an electrode surrounded by two insulators), leading to a thermal injury outside of the visual field.21
Trocars can have blunt, pyramidal, or conical tips. Pyramidal trocars require less force for insertion, but conical trocars may cause less tissue trauma during insertion.25 Several disposable trocars have spring-loaded tips that retract into a safety shield after the peritoneum is entered. If excessive force is used during insertion, the mechanism may not work properly; if the bowel is adherent to the fascia, injury will not be prevented. These should not be regarded as fool-proof safeguards.
Disposable instruments, in addition to their various designs, also have the advantage of always being sharp and readily available. Unfortunately, the cost to the hospital for disposable trocars and cannulas can range from $50 to $400. One study demonstrates that using disposable instruments only if reusable instruments were unavailable could markedly reduce the total cost of laparoscopy.26
If insufflation before trocar insertion is desired, several types of Veress needles are available, both reusable and disposable. A standard Veress needle is spring-loaded so that the dull inner stylet protrudes distal to the sharp outer needle on entering the peritoneal cavity. The Janicki needle has a vacuum sensor that can sense the negative pressure of the peritoneal cavity on placement.27 This sensor causes the light on the needle to change from red to green, signifying that the needle is in the peritoneal cavity.
For operative laparoscopy, carbon dioxide should be used for insufflation. High-flow insufflators should be capable of pumping 3–4 L/min of gas to a maximum of 10–15 mmHg in adults, 8–10 mmHg in children, and 6–8 mmHg in infants. High-flow insufflators pump carbon dioxide gas up to a rate of 40 L/min. These maintain adequate visualization in the setting of small leaks encountered throughout the case. After insertion of the Veress needle, a syringe with irrigation solution should be attached to the needle for aspiration of any blood or fecal material. This information should be appropriately documented at the time of surgery. At 15 mmHg, 94% of the abdominal volume is obtained in adults, and there is no change in the pressure required for trocar insertion up to 30 mmHg. Therefore, insufflation beyond 15 mmHg may be dangerous.28 Nevertheless, some gynecologists prefer higher intraperitoneal pressures of up to 25 mmHg for entry, followed by a return to 15 mmHg for the remainder of the procedure. An abdominal wall lifter can be used rather than insufflation for abdominal wall elevation, but visualization generally is poor with this instrument.
Should extraperitoneal insufflation occur, Kabukoba and Skillern29 describe a technique in which the laparoscope is advanced until it is 4 cm above the symphysis in the subcuticular tissue. The Veress needle then is introduced through a small incision just superior to the symphysis and directed toward the pouch of Douglas. The tip of the needle can be observed entering the fascia by the laparoscope. The trocar valve then is opened to release gas from the subcuticular tissue as the intra-abdominal pressure increases with insufflation.
The laparoscope contains fiberoptic bundles for image and light transmission. Laparoscopes ranging up to 11 mm are available. Although much work is being performed to improve the optics of smaller laparoscopes, most surgeons prefer 10-mm laparoscopes for operative procedures.
The camera equipment is composed of a charge-coupled device camera (CCD) and an output monitor. The CCD converts optical images into electrical signals. The information is transferred to the camera control unit, which transforms the electrical signals into optical images on the video monitor. Picture resolution is important; this term refers to the number of horizontal lines on the video monitor. These horizontal lines are composed of pixels, and the greater the number of pixels, the better the resolution.30
One of the disadvantages of laparoscopy is that video images are two-dimensional, and depth perception is difficult to judge. Undoubtedly, the use of three-dimensional laparoscopy will become widespread. A recent study demonstrates that surgeons were able to perform complex tasks more quickly and efficiently with three-dimensional compared with two-dimensional laparoscopy.31, 32 Also, the use of digital rather than electrical signals from the CCD will greatly improve images transmitted to the output monitor. The da Vinci Surgical System utilizes three-dimensional viewing and several manufacturers are producing high-definition optics, which will enhance the surgical setting. Validation studies of such high-end technologies have yet to determine improvement in outcomes.
Complications related to port closure have been reported with an incidence of 0.17–6.3%.33, 34, 35 Incisional hernias occur infrequently; however, because of the increased incidence encountered with larger ports, we recommend closure of those beyond 5 mm. Although the purpose is to secure facial defects, Richter's hernias may occur when large or small bowel is herniated through the peritoneum. These are especially devastating because they are more subtle, without a palpable mass, and may occur beyond the expected time frame for a facial herniation (approximately 10 days).
Approximating the fascia and peritoneum together is facilitated with closure devices such as the Carter-Thomason, which passes a free ligature through these layers under direct laparoscopic guidance. The diagnosis is primarily clinical and the presentation may be similar to that of an ileus, bowel obstruction, or wound hematoma. Computed tomography may also be useful in confirming the diagnosis. The small intestine, because of its caliber and mobility, herniates more frequently than the large bowel. Treatment is via laparoscopy or laparotomy and resection is seldom required.
SUTURING
Suturing can be performed with an intracorporeal knot, an extracorporeal knot, or a preformed knotted loop such as the Endoloop (Ethicon). Various types of needles exist specifically designed for laparoscopic suturing, but conventional needles also can be used.
Initially, straight needles were the only needles used laparoscopically. These needles are easier to introduce into the abdominal cavity but more difficult to control while suturing. Several modified straight needles have been developed to improve the situation.
Although introducing the needle through 10-mm ports is less time-consuming, back-loading through a smaller port is more cosmetic. A grasping device is placed into the abdomen through a smaller port and is used to stabilize the needle. The needle driver then is placed into the 10-mm port and the needle loaded. The tissue is stabilized with the grasper, and suturing can be accomplished.
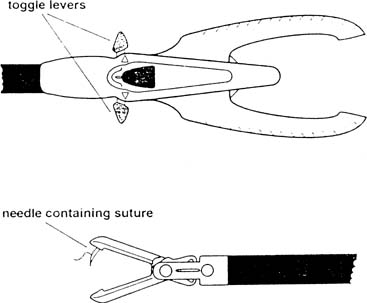
It can be difficult to place the needle in the correct location, because the abdominal wall prevents free movement of the needle driver because of a fulcrum effect. An alternative is the Endo Stitch (Autosuture, US Surgical) (Fig. 4), a laparoscopic suturing device with a curved needle that is sharp at both ends. The needle is passed back and forth between the two jaws by activating the forward or backward toggle lever for a grasping device to reload the needle before the next stitch. Suturing in tight spaces, approximating under tension, and obtaining larger purchases are not easily performed with these 10-mm devices; so, freehand suturing is recommended in these circumstances. The Delta Badia suturing device is similar in that it also has two jaws (Fig. 5); however, the needle is held in one jaw and is guided through a notch in the back jaw. A second instrument therefore is needed to help reload the needle.36, 37
It is technically more difficult to introduce a curved needle into the peritoneal cavity. Reich and associates38 describe a simplified method in which a 5-mm trocar is placed lateral to the deep epigastrics. The external and internal obliques and the transversalis fascia are penetrated to create a tract. The trocar and sleeve then are removed. The needle holder and suture are inserted through the tract as the needle follows. When the needle is in the peritoneal cavity, the needle holder is withdrawn and the sleeve replaced.
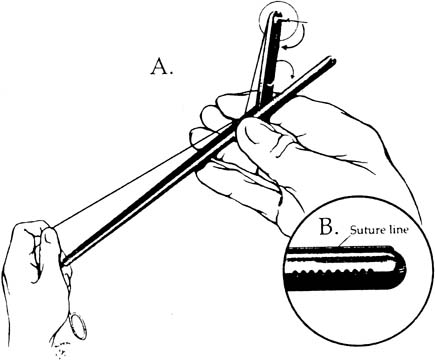
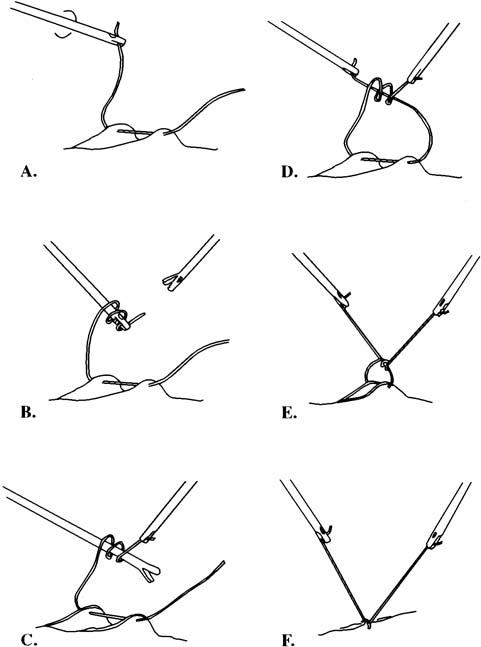
The needle can be loaded into the needle driver. The Cook needle driver (Cook OB/GYN, Spencer, IN) has a spring-loaded handle and a tip that holds the needle in the correct position. The Levine needle holder (Marlow, Willoughby, OH) has notched jaws for needle stability.39 Intracorporeal knots can be placed using the classic instrument tie technique. In an approach described by Topel,40 the left grasper, holding the suture, is turned twice, causing the suture material to wrap around the end of this instrument. The right grasper holds the free end. The left grasper then takes the other free end of the suture and pulls it through these loops. The same procedure is carried out again in the opposite direction to create a square knot (Figs. 6 and 7). After the needle is introduced into the peritoneal cavity and suturing takes place, an extracorporeal knot can be tied. In this instance, the suture is removed from the peritoneal cavity, and the throws are performed outside the body. Because a surgeon's throw does not tie-down well, a slipknot or several half hitches are recommended. A pretied extracorporeal suture also is available. Finally, the knot is tightened and pushed down with a knot-pusher.
|
In 1972, Clarke described the use of the Clarke ligator (knot-pusher) for suture ligation.41 A modification of his original instrument has been reported that can ligate and then cut the suture. The instrument has two grooved jaws that are used to push the knot down (Fig. 8). The jaws are opened to tighten the knot and, finally, the suture is cut with the jaws of the forceps.42 A similar method has been described (Autosuture, US Surgical) in which an endoscopic Babcock clamp rather than a knot-pusher is used to tighten the knot.43 Finally, the Endoloop, a device with a preformed knotted loop, can be used to ligate vascular pedicles or vessels (Fig. 9). After the Endoloop is slipped over the tissue, a grasper is used to stabilize the pedicle. The end of the plastic knot-pusher is broken and the knot cinched-down. Laparoscopic scissors can be introduced through the same port to cut the remaining suture. The Suture Assistant facilitates tying intracorporeal knots (Fig. 10). Keith needles may be introduced transcutaneously into the left upper quadrant of the peritoneal cavity to retract the sigmoid colon by its appendages. After securing these fatty structures, the needle is returned to the point of entry and a simple knot tied on the skin, giving enough tension to draw the mobilized sigmoid cephalad, allowing the surgeon to work in the deep cul de sac.
MICROLAPAROSCOPY
Developments in fiberoptic technology and the need to reduce the cost of health care have increased the interest in microlaparoscopy. With microlaparoscopy, a less invasive procedure can be performed under local anesthesia in an office setting.
With this procedure, an introducer is placed over a Veress needle before insertion. The microlaparoscope then can be inserted before insulation to verify appropriate placement. The introducer has an anchoring system and a side port for insulation. Various sizes of microlaparoscopes are available, including some smaller than 2 mm in diameter. The camera and light source systems used for standard laparoscopy also can be used for microlaparoscopy. Additional ports can be placed using the same Veress needle as a trocar.
Visualization with microlaparoscopy sometimes can be less than optimal. The picture on the monitor is approximately 30–40% smaller, and the depth of field is less than with a 10-mm laparoscope. Several studies compare the findings of a microlaparoscope with those of a 10-mm laparoscope. Feste15 performed 20 procedures with a 1.8-mm microlaparoscope in the operating room in conjunction with diagnostic laparoscopy. Occasionally, an endometrial implant hidden by an ovary was missed by the microlaparoscope, but overall the author was pleased with visualization. Bauer and Kupker44 used a 1.9-mm laparoscope to compare microlaparoscopy with conventional laparoscopy using a 10-mm laparoscope; results were identical in 27 of 28 procedures performed. One unilateral tubal malformation was not observed using small-diameter laparoscopy. In another study consisting of 52 procedures, the visualization of pelvic organs with 2- and 10-mm laparoscopes was judged to be excellent in 69.2% and 73.1% of the procedures, respectively.45 In 15.4%, visualization was poor secondary to adhesions with use of the microlaparoscope.
With microlaparoscopy, the need for general anesthesia may be eliminated. Adequate moderate sedation in the office setting, with the appropriate equipment and personnel is generally sufficient, and should a uterine manipulator be required, a paracervical block may be performed. Puncture sites also should be infiltrated with 1% lidocaine. For the comfort of the patient, only 500 mL of carbon dioxide gas should be used. Risquez and associates45 note that pneumoperitoneum was unpleasant to patients when the volume of gas exceeded 3 liters or if the intraperitoneal pressure exceeded 11 mmHg.
In one study, all procedures were completed 15 minutes after injection of the local anesthetic. Patients were monitored for 45 minutes and allowed to return to work 3 hours later. Total fees with the use of general anesthesia in a hospital setting were estimated to be $5000–7000 versus approximately $1125 with the use of microlaparoscopy.15 Bauer and Kupker44 report that patients undergoing this procedure without general anesthesia were highly satisfied. Despite the difference in operating room costs, the equipment itself is expensive and fragile. Proper care must be given to handling and sterilizing the equipment to counter the cost of purchasing should they break. Microlaparoscopy also has been useful for performing biopsies in patients with suspected malignancy.46
ULTRASONIC ENERGY (HARMONIC SCALPEL)
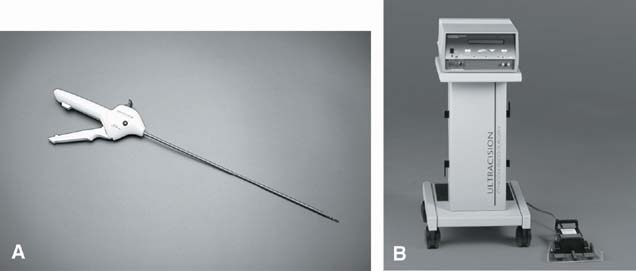
The ultrasonically activated scalpel uses ultrasonic mechanical energy to denature tissue protein into a sticky coagulum that seals blood vessels and bleeding tissues. The ultrasonic blade tip vibrates at 55,500 times per second over an area of approximately 100 mm. The mechanical energy breaks the hydrogen bonds that form the tertiary structure of proteins. The denaturization of the protein results in the protein coagulum that is capable of sealing vessels up to 5 mm in diameter without the charring and desiccation associated with electrosurgery and lasers.47, 48
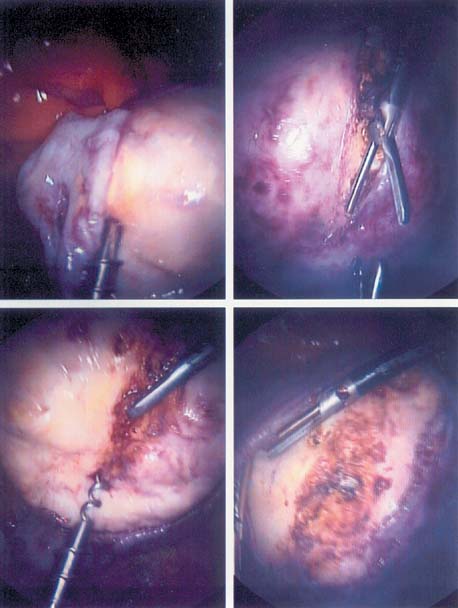
The Harmonic scalpel produces minimal lateral thermal damage, charring, carbonization, and bleeding leading to less macrophage activation and adhesion formation. It serves as a coagulant, cutter, and blunt dissector (Figs. 11, 12, and 13).
|
|
The greatest disadvantage of monopolar electrosurgery is that current is passed through the patient via the path of least resistance to the return ground electrode. As such, electrical capacitance to other viscera and remote thermal injury can occur. Monopolar current also produces smoke and char, which not only obscures the view and results in smoke inhalation by the surgeon but may also enhance the potential for adhesion formation. The advantage of this type of current is that it is an excellent cutting tool that is precise and can minimize superficial bleeding, which can obscure tissue planes. Bipolar electrosurgery, conversely, is an excellent modality for sealing vascular pedicles and controlling bleeding. There is less risk of remote thermal spread because the current passes between the two grasping electrodes. Like any other energy-based tool, the surgeon should be comfortable with the tool's use and aware of the limitations and associated risks. Harmonic instruments generate heat and plumes from water evaporation, and may not seal larger vessels. However, they can perform virtually every function needed for most gynecologic procedures. The latest version is the ACE, manufactured by Ethicon Surgical. The instrument is curved, and generates faster cycles, making it sufficiently facile. Olympus (SonoSurg Ultrasonic system) is the other primary harmonic scalpel manufacturer. Both are compatible with the da Vinci Surgical System.
ROBOTICS
Isaac Asimov's three laws of robotics are as follows: (1) a robot must not harm a human being or, through interaction, allow one to come to harm; (2) a robot must always obey human beings unless it is in conflict with the first law; (3) a robot must protect itself from harm unless it is in conflict with the first or second law. A surgical robot is defined as a “powered computer controlled manipulator with artificial sensing that can be reprogrammed to move and position a tool to carry out a range of surgical tasks. The goal of surgical robots is to assist the surgeon and not to replace him.”49
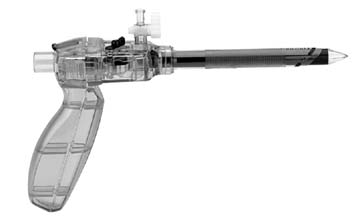
AESOP (Computer Motion, Goleta, CA) is the first FDA-cleared voice-controlled surgical robot. The surgeon's voice commands are previously recorded onto a voice card, which is inserted into the controller before surgery begins. AESOP allows the surgeon to directly control visualization and better use the operating room staff. A robotic device has been shown to more effectively manipulate and accurately control the video endoscope than a human assistant during a laparoscopic procedure50 (Fig. 14).
The ZEUS (Computer Motion) and da Vinci robotic systems allow a surgeon to remain seated at a workstation and operate handles designed to resemble conventional surgical instruments while the instrument tips remain inside the patient's body. ZEUS translates the surgeon's hand movement, then scales them into precise micromovements at the operative site. This system eliminates human hand tremors and allows the surgeon to scale natural hand movements into micromovements inside the body. This system uses a master–slave robot and is ideal for microsuturing techniques, such as laparoscopic tubal reanastomosis. ZEUS allows delicate work using microsurgical techniques with filtered tremors reduces surgeon fatigue and maintains well-controlled maneuvers.51
Telerobotic-assisted laparoscopic surgery allows an inexperienced surgeon to be mentored by an experienced surgeon at a remote site. It also permits a surgeon to operate on patients in sites without access to a surgeon. It is not far-fetched that in the future, telerobotics may play a major role in space expeditions, where surgeons on earth can operate on space settlers.52 Telerobotics can change the future of medicine. Remote rural areas can have access to surgeons operating from distant urban areas.
The da Vinci Surgical System (Intuitive Surgical) has "popularized" robotic-assisted procedures among urologists, and cardiothoracic and gynecologic surgeons. Seated in the console, the surgeon is afforded a three-dimensional image, several degrees of instrument articulation that standard laparoscopic instruments cannot perform, and ergonomics. The latest S-type utilizes four arms, one of which can serve as a tissue retractor. Various energy modalities have been adapted to include monopolar, bipolar, and harmonics. A camera port, two to three robotic ports (8 mm), and one or two surgical assist ports (varying sizes) to retract, irrigate, and pass sutures are used for any given procedure. Placement varies based on the surgical discipline or procedure. When proficient, the robot can be "docked" to the patient in under 5 minutes. For benign gynecologists, myomectomy and sacrocolpopexy, two procedures which require extensive suturing, are procedures of interest. The concept of reproducing that which would otherwise be done by laparotomy is maintained with this form of robotic surgery. There is a lack of randomized trials; however, a recent study comparing robotic-assisted and open myomectomy found that matched-patients who underwent the laparoscopic approach experienced less blood loss, fewer complications, and shorter hospital stay.53 Hospital and professional charges were significantly more costly in the robotic-assisted group. Its usefulness in gynecolgic oncology continues to evolve with lymph node dissection and radical hysterectomy as procedures that have been facilitated with the use of robotics.
INDICATIONS FOR OPERATIVE GYNECOLOGIC LAPAROSCOPY
Adnexal mass
NEWBORN
Ovarian cysts on antenatal ultrasound screening have become increasingly identified. Routine ultrasound has presented the clinician with a new dilemma: management of a fetal–neonatal ovarian cyst.54 Although most recommend taking a conservative (observation) approach, depending on the circumstances in which an ultrasound study identifies a septate or solid component, laparoscopic management also is advocated. Ovarian tissue should be salvaged if possible.55, 56
PEDIATRIC PATIENT
Although it is unusual for pediatric patients to have an indication for surgical intervention of an adnexal mass, laparoscopic oophorectomy in children has been reported. Ovarian torsion, hemorrhagic cysts, and the presence of a benign teratoma all were reported in a series from the University of Pennsylvania (Philadelphia).57 In each case, oophorectomy was successfully completed laparoscopically in pediatric patients. The postoperative course was characterized by a short recovery period. The authors report that it is technically an easy procedure, with the benefits of excellent visualization of the entire lower abdomen and pelvis, including the opposite ovary.
In a series reported by Schier and Wildschmidt,58 225 pediatric patients with ill-defined abdominal pain underwent laparoscopy. Their findings resulted in a laparoscopic approach that included appendectomy, lysis of adhesions, cyst resection, removal of Meckel's diverticula, and herniotomies. The patients ranged from neonates weighing 1850 g to teenagers who were aged 15 years, with the average age of 8 years. Entry via open laparoscopy is recommended given the close proximity of the large vessels to the skin in younger patients. The authors conclude that from the perspective of completeness, cosmetic result, hospitalization time, and return to normal activities, a laparoscopic approach was the most feasible.
PEDIATRIC PATIENT, ADOLESCENT, OR ADULT
In a 12-year experience (1980–1991), Canis and coworkers59 in France noted that the laparoscopic approach allowed for peritoneal cytology, ovarian and peritoneal inspection, cyst aspiration, and endocystic examination. Of the 757 patients, 819 masses were managed by laparoscopy; if a malignancy was identified, immediate laparotomy was performed. The authors conclude that laparoscopic management is feasible for well-screened patients, but patients should be prepared to undergo possible immediate laparotomy. Ovarian conservation and future fertility are of great concern, especially in the setting of a suspicious pelvic mass. Although most masses encountered in adolescents are benign (follicular cyst, mature teratoma), a higher incidence of malignancies will be detected in childhood. Conservative laparoscopic staging in the setting of early disease may be performed by oncologists trained in advanced laparoscopy, and future childbearing potential will be improved.
ADULT
A laparoscopic approach often is feasible for correcting an ovarian cyst. Depending on the preoperative evaluation, including ultrasonographic assessment, laparoscopy may be the most practical approach. Although specific indications for operative intervention must be predicated on the individual clinical circumstance, the endoscopic approach may result in evaluation alone, cyst aspiration, ovarian cystectomy, or oophorectomy. Placement of the incisions (ports) is crucial for facilitating the procedure, as is a proactive approach toward the prevention of bleeding. Use of coagulation to control bleeding is appropriate and often all that is necessary. Suture placement in the ovary should be avoided to minimize adhesion formation.
Mature teratoma
In a series of 70 patients treated laparoscopically for ovarian mature teratoma, a conservative (ovary-preserving) approach was feasible in 60 patients.60 The remainder required salpingo-oophorectomy. The authors conclude that when performed by experienced surgeons, laparoscopic removal of an ovarian mature teratoma is safe and is an approved alternative to laparotomy. The obvious concern is possible rupture of the ovarian cyst. Although effort may be made to remove the cyst intact and to place it in an endoscopic pouch to prevent spillage, the desired result may not always occur. In case of spillage, intracystic contents should be contained if possible, followed by copious peritoneal cavity irrigation. The contents of mature teratomas have been shown to be strongly adhesiogenic;61 therefore, cyst rupture should be avoided at all costs. If rupture occurs, irrigation will lessen the risk of developing subsequent adhesions. The opposite adnexa always should be evaluated for bilateral disease.62
Ovarian neoplasm subsequently noted to be malignant
The frequency of determining a malignant ovarian mass with a laparoscopic approach is a question of much interest. This point was pursued in a countrywide survey in Austria.63 Of the 54,198 laparoscopies, 16,601 were performed for adnexal masses; furthermore, 108 cases of ovarian tumors subsequently found to be malignant were identified. Of the 108 cases, 20 were treated laparoscopically, 22 had immediate laparotomy, and the rest underwent a delayed laparotomy, ranging in time from 3 to 1415 days. Thirteen patients died of tumor progression. Preoperative risk stratification will minimize the risk of performing an inappropriate procedure. Age of the patient, transvaginal ultrasound, and CA-125 may all be useful in the preoperative assessment. Should suspicion be high, an oncologist should be consulted before embarking on a difficult case. Management of a suspicious mass should be precise and measures should be taken to avoid content release. Surgical pathology should be present for frozen sectioning to determine if staging is indicated at the initial procedure. Consent should include all possible contingencies to avoid another procedure and exposure to general anesthetics. It is also imperative that staging be performed as soon as possible once malignancy is diagnosed. As more oncologists are trained in endoscopy, laparoscopy may eventually replace laparotomy for appropriate staging and treatment of several gynecologic malignancies.
Aspiration versus excision
Controversy continues regarding whether laparoscopic cyst excision is satisfactory compared with aspiration. In a series of 100 patients younger than age 40 years with a cystic adnexal mass, cyst excision using a stripping technique was compared with laparoscopic cyst aspiration. Four recurrences (4%) were observed in the group having undergone laparoscopic cyst excision versus 84% in the aspiration group. The authors determined the treatment of choice to be enucleation of the adnexal mass.64 Follicular cysts generally resolve spontaneously; however, endometriomas and benign cystic neoplasms recur unless the entire cyst is destroyed or excised.
Polycystic-appearing ovaries
Traditionally, ovarian wedge resection has been advocated for selected patients with infertility. With the advent of a minimally invasive approach, laparoscopic treatment of polycystic ovaries, often termed the whiffle ball procedure, has taken its place in the endoscopic armamentarium; however, the importance of proper patient selection continues to be advocated. Ideally, the patient should have no response to menotropins, preceded by clomiphene citrate. Also, proper counseling (informed consent) about the potential for ovarian adhesion formation is important and must be clearly documented.65, 66, 67, 68 The approach usually involves ovarian electrocauterization or use of a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser or photocoagulation. Other options include ovarian punch biopsy. Effort to maintain hemostasis must be made to minimize adhesion formation. The endocrinologic changes appear to parallel those of wedge resection.66 Meta-analysis of six randomized trials with patients having polycystic ovarian syndrome (PCOS) have been reported.69 The mean body mass index ranged from 23 to 29.5 kg/m2 with the following characteristics: clinical features of PCOS, ultrasound evidence, and endocrinological abnormalities consistent with PCOS. All did not ovulate with clomiphene treatment. In those who received gonadotropin therapy, they did not conceive. The trials included arms with ovarian drilling by electrocautery compared with human menopausal gonadotropin (HMG) therapy, ovarian drilling with HMG, or follicle-stimulating hormone alone. If no ovulation occurred after drilling, patients were treated with clomiphene or gonadotropins.
The results noted no statistical difference with ovarian drilling compared with gonadotropin therapy alone at 6- and 12-month follow-ups. There was no significant difference in ovulation rates, pregnancy rates or pregnancy outcomes. There was evidence for lower multiple gestation rates after drilling clomiphene treatment. Continued concern is expressed for adhesion formation after drilling. Currently, laparoscopic ovarian drilling cannot be recommended in preference to clomiphene as a primary form for treatment for PCOS.
Adnexal torsion
Traditionally, adnexal torsion has been associated with the potential for dislodging an embolus, but the literature has not substantiated this concept. Current practice involves untwisting the adnexa (fallopian tube or ovary alone) and determining whether viability is present. Suggested approaches range from visual inspection of the tissue to intraoperative assessment with ultrasonography, including color Doppler studies. Depending on the operating room equipment available and the surgeon's training, once the decision is made to approach the adnexa conservatively, untwisting, followed by removal of any underlying diseased tissue such as an ovarian cyst, should be considered. Performing oophoropexy to the ipsilateral sidewall or round ligament may be performed to avoid recurrence. In a series of seven girls with adnexal torsion of varying degrees, all treated laparoscopically, six were noted to have a simple follicular cyst and one had a dermoid.70 Detorsion was associated with re-establishment of tissue viability. Correction of the underlying disease then occurred, with resultant preservation of ovarian tissue.71 Pena and coworkers72 showed that Doppler sonogram is not sensitive in the diagnosis of ovarian torsion. Lack of flow to the ovary is diagnostic of torsion; however, 60% of ovarian torsions have normal flow.
Prophylactic oophorectomy in cancer prevention
The question often is debated as to whether the ovaries should be removed during other pelvic operative procedures to prevent ovarian cancer. A study by Averette and Nguyen addresses this question.73 From a theoretic perspective, 7% of patients with ovarian cancer have a positive family history, 3–9% of whom have a hereditary cancer syndrome. In addition, there is a direct genetic linkage of familial carcinoma syndromes, being associated with a 50% lifetime risk of ovarian cancer. The authors recommend that prophylactic oophorectomy be performed for women with familial cancer syndromes after childbearing or by age 35–40 years at the latest. Patient selection and presurgical informed consent remain important in preventing ovarian cancer in a specific patient, recognizing that lifelong estrogen replacement therapy will be necessary.73 More recent evidence suggests that preserving ovaries, perhaps even in menopausal women, at time of other gynecologic surgery (such as hysterectomy) may enhance long-term survival. These recommendations are based on mathematical models that indicate a tendency towards more cardiovascular events and hip fractures in patients who elected to undergo prophylactic oophorectomy.74 Although prophylactic oophorectomy is still debatable in patients with no known heritable risks, those who are at risk should undergo counseling by an oncologist and geneticist prior to surgery.
GYNECOLOGIC CANCER AND LAPAROSCOPY
Despite rapid advances in operative laparoscopy, the standard of care for many gynecological malignancies remains laparotomy. As operative laparoscopy becomes more integrated in resident and fellow training, laparoscopic staging will likely change the course of surgical management for many of these patients. The rapid healing and return to normal activities makes laparoscopic staging a more attractive option, especially because postoperative adjuvant therapy may be administered more expeditiously than when compared with laparotomy.
Ovarian cancer
The most common uses for laparoscopy in patients with presumed or known ovarian malignancies include: (1) evaluation of the adnexal mass; (2) staging of apparent early stage disease; and (3) obtaining a second look.
Rupture of a malignant ovarian mass during laparoscopy is a major concern. Although the prognosis of an otherwise stage Ia ovarian cancer may not be affected by rupture, the upstaging to Ic dictates postoperative adjuvant chemotherapy. Rupture before surgical intervention clearly portends a more grim prognosis. The laparoscope affords excellent visualization of the pelvic and abdominal peritoneum. Random biopsies and omentectomy can be easily performed; however, thorough evaluation of the bowel and its mesentery can be more difficult with laparoscopic instruments compared with laparotomy. Endobag should be used to remove an intact cyst during laparoscopy if suspicion is high for malignancy. Using an Endobag extractor (Storz, Germany) can facilitate mass removal via colpotomy or the port site itself. The skin and subcutaneous tissues should be irrigated after removal and instruments such as the suction irrigator tip replaced if used in the process of removal.
Cervical cancer
Cervical cancer is staged clinically. The surgical management is limited to early stages IIA or less. The patients usually undergo radical hysterectomy and bilateral pelvic lymphadenectomy. Advanced stages are managed with radiotherapy with or without chemotherapy. There are two potential approaches to laparoscopic management of early cervical cancer. First is the modified Schauta radical vaginal hysterectomy and total laparoscopic radical hysterectomy with bilateral pelvic lymphadenectomy (LARVH). The former is seldom used in the United States because of limited training and experience. More than 250 patients worldwide have undergone LARVH. The average operating time is 93 minutes.75
Canis and Nezhat and their coworkers first described total laparoscopic radical hysterectomy in 1992.76 The average length of their procedures was 8 and 7 hours, respectively.
In 1996, Spirtos and coworkers described a series of ten patients who successfully underwent laparoscopic radical hysterectomies with aortic and pelvic lymphadenectomy. The average operating time was 253 minutes, with an average blood loss of 300 mL.77 Other larger series have been described, but no randomized trials specific to cervical cancer have been conducted. Nevertheless, laparoscopic radical hysterectomy for early stage cervical cancer is expected to increase in the United States. Laparoscopic radical trachelectomy may be considered in patients who have cervical neoplasia after hysterectomy.
Endometrial cancer
The currently accepted surgical management of endometrial cancer is total abdominal hysterectomy (TAH)-bilateral salpingo-oophorectomy (BSO) with peritoneal washing and retroperitoneal lymph node sampling. Bajaj reports a retrospective assessment of 28 patients who had laparoscopic surgical staging with BSO and then total vaginal hysterectomy. In the series, 23 patients (82%) had stage I disease, two (7%) had stage II, and three (11%) had stage III disease. Complications included herniation through a 5-mm port site, requiring small bowel resection; a fatal myocardial infarction occurred 10 days postoperatively.76
The Gynecologic Oncology Group performed a randomized stage III trial comparing laparoscopic-assisted surgical staging and staging using laparotomy in the management of women with early endometrial cancer. Preliminary results were presented in abstract form and suggested that approximately 76% of patients with stage I–II disease could successfully undergo laparoscopic staging without a difference in short-term outcomes. Conversion to laparotomy occurred in 24% of patients and the authors recommended this be an essential component when unable to completely stage laparoscopically.77
LAPAROSCOPIC TUBAL REANASTOMOSIS
Laparoscopic surgery has been used to accomplish tubal reanastomosis in patients requesting reversal of sterilization. In a pilot study involving five patients who desired reversal of sterilization, a laparoscopic approach was associated with infiltration of 5 mL of a dilute vasopressin solution in the occluded segment of the tube.78 The tubes next were incised with laparoscopic scissors, and proximal segment patency was established with an intrauterine injection of dilute indigo carmine. Cannulation of the proximal segment was accomplished under hysteroscopic guidance using a catheter in a manner similar to that of tubal cannulation for proximal occlusion. The serosa of the distal tubal segment was grasped, and the incised portion cannulated with a 3-French catheter wire guide, with resultant proximal and distal segments of the tube being coapted. The mesosalpinx was approximated with 5-0 polydioxanone suture, which was laparoscopically tied. Patients received prophylactic antibiotics.
If the patient is not pregnant within 3 months after surgery, a hysterosalpingogram (HSG) is obtained. Of ten fallopian tubes laparoscopically reanastomosed by Katz and Donesky,78 postoperative HSG revealed four patent tubes and four occluded tubes; one patient did not undergo HSG. In another laparoscopic tubal anastomosis series reported by Dubuisson and Swolin,79 a single suture placed at the 12-o'clock position on the antimesenteric border accomplished reanastomosis in four patients, three of whom had postoperative patency on HSG and one who had no HSG performed. The authors attested to the efficacy of a single-suture laparoscopic approach to anastomosis. A report of fertility after laparoscopic microsurgical tubal reanastomosis involved 54 patients who underwent the procedure in Korea;80 the overall pregnancy rate was 77.5% (38 of 49 actively attempting a pregnancy). Twenty-nine patients delivered at term. At the time of publication, seven pregnancies were ongoing (36 of 54). It was concluded that pregnancies were noted primarily within the first 12 months after laparoscopic microsurgical tubal reanastomosis. The technique included a 6-0 polydioxanone suture being placed over the mesosalpinx, followed by reapproximation of the muscularis with the use of 7-0 polydioxanone or 8-0 Vicryl (Ethicon). The sequence was first a 6-o'clock stitch, followed by 12-o'clock reapproximation, and then the 3- and 9-o'clock positions also were reapproximated. This study demonstrates an overall pregnancy rate of 77.6%, with a 2.6% ectopic pregnancy rate. The authors conclude that a laparoscopic approach is associated with a high pregnancy rate. Follow-up HSG was recommended 12 months after the minimally invasive procedure.
Lee and coworkers from Taiwan81 report on laparoscopic rescue after tubal anastomosis failure. In this case involving a 33-year-old woman with a previous sterilization, restoration was established using minilaparotomy with isthmic end-to-end anastomosis of the fallopian tubes bilaterally. Tubal patency was noted on HSG 6 months after surgery. The previously occluded site was reanastomosed, and patency was established with intrauterine injection of dye. The occluded section of fallopian tube was segmentally resected and reanastomosis performed. The authors conclude that laparoscopic rescue with repeat isthmic–ampullary anastomosis and lysis of adhesions was feasible and could be an alternative to assisted reproductive technology.
In 1999, Bissonnette and colleagues82 reported an observation prospective laparoscopic tubal reanastomosis series. Laparoscopic tubal reanastomosis was performed with a one-suture technique at the 12-o'clock position. There were 69 isthmic–isthmic, 16 isthmic–ampullary, 12 cornual–isthmic, and five ampullary–ampullary anastomoses. The mean operative time was 71 minutes. Eight patients had bilateral tubal obstruction on the postoperative HSG. Sixty-nine patients (70%) conceived. Sixty-four (65.3%) had intrauterine pregnancies. Fifteen (21.7%) had spontaneous abortions. Five (7.2%) had ectopic pregnancies. This study demonstrates that laparoscopic tubal reanastomosis can be performed on an outpatient basis with similar results to minilaparotomy microsurgical reversal of tubal sterilization.
Robotic-assisted laparoscopic microsurgical tubal reanastomosis
In 2000, Falcone and associates51 from the Cleveland Clinic reported a prospective pilot study on microsurgical tubal anastomosis. The study used the ZEUS robotic system (Computer Motion) in performing laparoscopic microsurgical anastomosis in humans. The United States FDA approved this study with ten patients. The inclusion criteria included a normal semen analysis in the partner and a HSG that showed at least 1 cm of proximal tube.
The ZEUS system has three remotely controlled robotic arms, allowing manipulation of two laparoscopic instruments and the camera by one surgeon. The surgical instruments are controlled by two handles housed in a mobile console positioned anywhere in the operating room or in a different location outside the operating room. A computer controller translates the surgeon's movements from the handle to the robotic arms. This system is ideal in eliminating tremors and unintended hand motions routinely seen in microsurgery.51
A two-layered closure was used for all tubes. Four stitches of 8-0 polygalactin sutures were used for each layer. Chromotubation at the end of the procedure showed patency in all tubes that underwent microanastomosis. Postoperative HSG showed 89% patency rate. Open laparotomy was not needed in any patient. The 1-year pregnancy rate was 50%. The mean time required to complete both tubes was 159 ± 33.8 minutes.51
A small trial recently evaluated the use of robotic-assistance compared to outpatient minilaparotomy for tubal reanastomosis and found that the outcomes were similar (hospitalization times, pregnancy and ectopic pregnancy rates). Patients who underwent a robotic procedure had a shorter return to normal activity, but incurred a significant difference in cost.83
MYOMECTOMY AND CRYOMYOLYSIS
When a symptomatic leiomyoma is identified, there are several surgical alternatives. Abdominal myomectomy had been the traditional approach. Laparoscopic myomectomy has proven to be effective; however, the potential for uterine dehiscence in pregnancy has been reported.84 The reader should keep in mind that obstetric complications after abdominal myomectomy have not been consistently reported for decades, and since the laparoscopic approach is relatively new in comparison, it is easier to review its outcomes. Some have suggested that the use of monopolar energy on the uterine serosa and underlying myometrium (necessary to perform this procedure laparoscopically and maintain hemostasis) results in unseen thermal injury, predisposing to less than ideal healing. This could explain uterine rupture subsequent to removal of a pedunculated myoma.85 Surgeons should utilize the CUT setting if choosing monopolar energy, and consider suturing the bed even if the myoma is pedunculated. Harmonic energy may also be used for incision and enucleation. Choice of energy should be left to surgeon preference, but whichever is selected should be used with caution and in a sparing fashion to minimize unnecessary tissue trauma. The largest laparoscopic series to date, which included over 2000 patients, reported a complication rate of 2%.86 Ultrasonic energy can be safely used as an alternative, but surgical principles that would otherwise be utilized during open surgery should be maintained. The all-inclusive postoperative pregnancy rate was approximately 70%, and only one uterine rupture was reported.
Criteria for performing laparoscopic myomectomy vary based on experience, but published literature suggests the following are risks for laparoconversion: myoma size >5 cm, intramural and anterior location, and gonadotropin releasing hormone agonist (GnRHa) use.87 Dilute vasopressin (Pitressin) injected in the myoma bed is used to achieve vasoconstriction. A monopolar electrode (CUT setting) or harmonic scalpel is then used to incise the serosa and myometrium to the level of the myoma. The myoma is stabilized with a myoma screw or tenaculum, and the myoma is enucleated using traction, counter traction, and an energy source to minimize intraoperative bleeding. A morcellator then is used to extirpate the myoma. Intracorporeal running suture or interrupted sutures using 00 caliber suture can be placed in one to three layers, depending on the original location of the myoma. Various adhesion barriers exist, and can be used laparoscopically to minimize the risk of postoperative adhesions. This procedure strongly resembles that described by Miller in 1996 with the morcellator modification.88 This provides faster removal of the myoma. The operation time is approximately 2.5 hours, and estimated blood loss is approximately 100 mL (Fig. 15). Extensive iatrogenic adenomyosis after laparoscopic myomectomy has been reported in a patient who did not have suturing of the myometrium layers.89 The introduction of the da Vinci Surgical System may allow surgeons to virtually reproduce the procedure identical to how it is performed by laparotomy. Obstetric outcomes will ultimately prove or disprove the utility of this facile device. Until then, it is an acceptable option for those who are comfortable with both laparoscopy and the use of this system.
Laparoscopic myolysis, although not frequently utilized, has been proposed using various energy forms (diathermy, laser, freezing probe). When using a cryoprobe, the process requires placement of a 5-mm laparoscopic port, followed by monopolar point coagulation (5 mm), preceded by injection of dilute vasopressin at the point of insertion of the cryoprobe. On completion of the monopolar segment of the procedure, a cryoprobe is placed directly into the leiomyoma. Next, a cryothermal unit is engaged with production of a 180°C freeze. This is followed by a thaw and a second freeze of the leiomyoma. On sonography, an ice ball is identified, usually extending several centimeters from the probe. The procedure then is completed, the probe is removed, and a hemostatic agent is placed into the track. This procedure has not withstood the test of time and is not used in current practice.
PELVIC INFLAMMATORY DISEASE
Pelvic inflammatory disease occurs in all sectors of society. The diagnosis may be in question, depending on the presentation. A laparoscopic method of evaluating acute pelvic pain may prove to be efficacious, especially when the diagnosis is not clearly discerned and a differential diagnosis is required. The literature attests to laparoscopic treatment for tubo-ovarian abscesses in a series by Henry-Suchet and colleagues90 in which 50 women with tubo-ovarian abscesses received a dose of parenteral antibiotics followed by surgical assessment and treatment, including laparoscopic resection of the acute tubo-ovarian complex. The procedure also included lysis of adhesions and extensive peritoneal cavity lavage with an antibiotic-containing solution. The authors report a rapid recovery in 45 of 50 patients, with complete dissipation of the mass within several days postoperatively, and recommend the laparoscopic approach as being feasible. Other authors also attest to the efficacy of a laparoscopic approach. Reich and Shaw91 report on a series of 48 women with tubo-ovarian abscesses and again note the effectiveness of early laparoscopic intervention. Cultures can be obtained from the peritoneal cavity, and patients complete a course of parenteral antibiotics postoperatively.
LAPAROSCOPIC HYSTERECTOMY
In 1989, Reich and associates first described LAVH.92 This procedure is indicated for patients who otherwise would be unable to undergo a vaginal hysterectomy. Indications for LAVH include known leiomyomata, suspected pelvic adhesions, advanced endometriosis, and pelvic mass. The procedure has many advantages over abdominal hysterectomy. Patients have smaller incisions and a reduced need for postoperative analgesics. They also have a shorter hospital stay and can return to their previous level of activity at a faster rate. Unfortunately, this procedure usually takes a longer time to complete, resulting, of course, in a higher cost to the patient.
In a prospective randomized study, ten patients underwent either an abdominal hysterectomy or LAVH.93 The latter group had a shorter hospital stay (1.3 versus 4.5 days) and a more rapid recovery (3 versus 5 weeks), but the duration of surgery was longer (160 versus 102 minutes).
Summitt and associates94 randomly assigned 56 women to undergo LAVH with endoscopic staples or a vaginal hysterectomy. All of the patients who underwent a vaginal hysterectomy, and 27 of 29 patients who underwent LAVH had no complications. One of the two complications that occurred was injury to the right inferior epigastric artery, which could not be repaired laparoscopically. In the second patient, a cystotomy was performed, which was closed with laparoscopic suturing. The mean operating time for the LAVH group was 120 minutes versus 64 minutes for the vaginal hysterectomy group; 53 of the 55 patients completing surgery were discharged home in 12 hours. Patients undergoing LAVH required significantly more pain medication. The postoperative course for both groups of patients, however, was similar. The most glaring difference in these two procedures was the cost: the mean hospital charge was $4891 for a vaginal hysterectomy and $7905 for LAVH.
In a retrospective review comparing 70 patients undergoing LAVH, vaginal hysterectomy, and abdominal hysterectomy, Doucette and Scott95 report similar findings. Operating times were shorter, which may reflect the surgeon's level of training. However, LAVH still took the longest amount of time to perform at 80 minutes. Vaginal hysterectomies were performed in an average of 40 minutes, abdominal hysterectomies were performed in an average of 50 minutes. Complications were similar in all groups. The LAVH group required the least postoperative anesthesia. The average hospital costs were $5835, $3946, and $3414 for LAVH, vaginal hysterectomy, and abdominal hysterectomy, respectively. Another study compares the cost of surgery for 50 patients undergoing LAVH versus 46 patients undergoing a total abdominal hysterectomy (TAH) in a health maintenance organization.96 The cost of disposable instruments used during LAVH was $1250. It was estimated that the reduction in hospital stay (by 2.2 days in the LAVH group) netted a cost savings of $629. The authors acknowledged, however, that the cost of hospital stay could not be estimated easily because the patient was not billed in a fee-for-service manner.
Nezhat and associates97 found that the cost of performing LAVH with a stapling device was significantly higher ($7161.66) than the cost of a vaginal hysterectomy ($4868) or an abdominal hysterectomy ($4926.80). Although the patients undergoing an abdominal hysterectomy had a much higher postoperative pharmacy bill secondary to a longer hospital stay, this failed to offset the cost of instruments and the longer time in the operating room. The cost reported in the literature for LAVH ranged from $1051 to $11,931.98, 99, 100
Laparoscopic-assisted vaginal hysterectomy technique
The technique for LAVH requires the patient to be placed in the dorsal lithotomy position in stirrups. The bladder is emptied, and a preoperative antibiotic is administered for infection prophylaxis 30 minutes before the start of the procedure. After a pneumoperitoneum is established, a 12-mm port is placed inferior to the umbilicus. Placement of at least three other ports is advisable: one in the midline and two lateral in the lower abdomen. Placement of two 10-mm ports lateral to the rectus muscles makes it possible to put the laparoscope in the lateral ports and place the stapler through the umbilical port. By following this procedure, the surgeon is less likely to injure the ureter secondary to excessive lateral placement of the stapling device. Initially, the course of the ureter should be identified. Parker101 advocates doing this at the beginning of the procedure before edema occurs, obscuring the view of the ureter. The ureter should be traced throughout its course, starting at the pelvic brim. At the last point where the ureter can be identified, the peritoneum is elevated and an incision is made over the ureter for easier identification later during the procedure.
Hemostasis of all pedicles is achieved with bipolar energy, surgical staples, or clips. The procedure should mimic that which is typically performed by an open incision. Round ligaments are coagulated and transected, and the vesicouterine serosa deflected anteriorly and uterine serosa posteriorly. The infundibulopelvic ligaments are identified and ligated if the ovaries are to be removed, otherwise the utero-ovarian pedicles are transected for ovarian preservation. The bladder then is sharply and bluntly dissected off the lower uterine segment and cervix. The uterine vessels are skeletonized, coagulated, and transected if performed laparoscopically. At this point, many surgeons prefer to continue the remainder of the case vaginally. However, if the dissection of the uterosacral and cardinal ligaments is accomplished laparoscopically, the location of the ureters must be kept in mind. As Parker102 points out, the ureter at the level of the uterine artery is 15 mm lateral to the cervix, and most stapling devices are 12 mm wide; thus, the ureter is in close proximity. After closure of the vaginal cuff laparoscopically or vaginally, the pelvis is reinsufflated, and all pedicles are observed for hemostasis. Cephalad traction of the uterus with a uterine manipulator will allow for easier colpotomy performed laparoscopically, and will displace the ureters in a more lateral and posterior direction.
Variations on this procedure have been reported in the literature.100, 103 One group reports a modified technique they call a laparoscopic completion of a vaginal hysterectomy.104 In this study, all procedures were approached vaginally. The uterosacral and cardinal ligaments were clamped, transected, and ligated in the usual fashion. Both the anterior and the posterior cul-de-sacs were entered. If the procedure could not be performed vaginally, a uterine manipulator with an inflatable balloon was placed. The manipulator also had hooks that attached to the cervix to secure the specimen. The rest of the procedure was completed laparoscopically. The authors used this approach to help detect patients who were candidates for LAVH.
Wood and Maher105 report a laparovaginal hysterectomy. In this procedure, a 10-mm umbilical incision was made, and the laparoscope and sleeve were placed in this incision. Next, a 2-cm midline incision was made in the lower abdomen for placement of an abdominal wall elevator. Then, another 2-cm incision was made in the midline below the abdominal wall elevator. Through this incision, the surgeon's finger could be inserted for blunt dissection and ligation of pedicles. Two additional 5-mm lateral ports were placed adjacent to the rectus muscles for introduction of additional laparoscopic instruments. This procedure was performed on three patients in 120, 60, and 90 minutes, respectively.
Henley and Wells describe a modification of the standard LAVH.106 After the uterine arteries were ligated, they report using blunt dissection to develop a plane between the cardinal ligament and the uterus. The cardinal ligament was retracted laterally, and the small perforating vessels traversing this plane were cauterized. The advantage of this technique was that less devascularized tissue was left in the abdomen, and injury to the ureter decreased because it fell away laterally, along with the cardinal ligament.
Several studies have found that the complications after LAVH are comparable with those of abdominal and vaginal hysterectomy.93, 94, 107 A retrospective analysis of 45 patients reveals that 11% of the complications were operative, including one bladder perforation, two superficial epigastric artery perforations, and two procedures in which the patients had subcutaneous emphysema.108 Other complications were secondary to anesthesia in 7%, nursing in 4%, and postoperative complications in 16%; the remainder were caused by equipment failure (56%). The most common equipment failure reported is bipolar cautery dysfunction. Bladder perforation was deemed the most severe complication by the authors. Of the 45 patients, 40 had a supracervical hysterectomy; therefore, complication rates may be even higher in patients undergoing a complete hysterectomy. No comment was made as to how many patients underwent oophorectomy.
Many of the data evaluating outcomes and complications are difficult to interpret. There was a lack of consistency before 1993 in defining the portion of the procedure that was completed laparoscopically. A classification now defines a type I LAVH as dissection up to, but not including, the uterine artery;109 type II dissection includes bilateral or unilateral uterine artery and vein occlusion; type III dissection includes a type II dissection plus dissection of a portion of the cardinal and uterosacral ligament, either bilaterally or unilaterally; and type IV dissection includes the aforementioned plus total transaction of the cardinal and uterosacral ligament, either bilaterally or unilaterally (Table 5).
Table 5. Classification system for laparoscopically directed and assisted total hysterectomy*
| Type 0 | Laparoscopically directed preparation for vaginal hysterectomy |
| Type I† | Dissection up to but not including uterine arteries |
| IA | Ovarian artery pedicles only |
| IB | A + anterior structures |
| IC | A + posterior culdotomy |
| ID | A + anterior structures and posterior culdotomy |
| Type II† | Type I + uterine artery and vein occlusion, unilateral or bilateral |
| ILA | Ovarian artery pedicles(s) plus unilateral or bilateral uterine artery and vein occlusion only |
| IIB | A + anterior structures |
| IIC | A + posterior culdotomy |
| IID | A − anterior structures and posterior culdotomy |
| Type III | Type II + portion of cardinal–uterosacral ligament complex; unilateral or bilateral, plus: |
| IIIA | Uterine and ovarian artery pedicles with unilateral or bilateral portion of the cardinal–uterosacral complex only |
| IIIB | A + anterior structures |
| IIIC | A + posterior culdotomy |
| IIID | A − anterior structures and posterior culdotomy |
| Type IV† | Type II + total cardinal–uterosacral ligament complex; unilateral or bilateral, plus: |
| IVA | Uterine and ovarian artery pedicles with unilateral or bilateral detachment of the total cardinal–uterosacral ligament complex only |
| IVB | A + anterior structures |
| IVC | A + posterior culdotomy |
| IVD | A − anterior structures and posterior culdotomy |
| IVE | Laparoscopically directed removal of entire uterus |
*The classification system describes the portion of the procedure completed laparoscopically.
†A suffix “0” may be added if unilateral or bilateral oophorectomy is performed concomitantly (e.g., type 10A).
An LAVH is an acceptable alternative to abdominal hysterectomy in appropriately chosen patients. Advantages include less patient discomfort and a faster recovery period. However, most studies report that it is a more expensive procedure because of the use of disposable instruments and longer operating times.
Total laparoscopic hysterectomy using the harmonic scalpel
In 1999, Winter110 reported his experience with TLH using the Harmonic LCS (Ethicon). The entire hysterectomy, including transection of the uterine vessels and opening/closure of the vaginal vault, was performed laparoscopically. The Harmonic scalpel was used for coagulation and cutting, including transection of the uterine vessels and opening of the vagina. The 10-cm initially was used for the first half, and then the 5 cm for the second half of the procedure. In addition, the Rumi system uterine manipulator, Kon cup vaginal fornices delineator, and colpopneumo occluder were used (Cooper Surgical, Shelton, CT). Average hospital stay was 1.8 days for TLH, 1.9 days for LAVH, 2.3 days for vaginal hysterectomy, and 2.6 days for TAH. No increased morbidity was noted in comparison with LAVH, vaginal hysterectomy, or TAH. The Harmonic scalpel costs $325 per case (slightly higher for the Ethicon ACE) compared with $1600 for Endostaples. In summary, the Harmonic scalpel is an effective instrument that can facilitate TLH for relatively small uteri.
COLPOSUSPENSION AND SACRAL COLPOPEXY
In 1948, Marshall and associates111 described a procedure for correcting urinary incontinence that elevated the urethrovesical junction. It restored continence in 82% of patients. In this procedure, the periurethral fascia was sutured to the symphysis, predisposing the patient to osteitis pubis. Burch modified this procedure by elevating the urethrovesical junction and suturing the paraurethral fascia to Cooper's ligament rather than the symphysis pubis. The Burch urethropexy is considered the gold standard for surgical treatment of genuine stress urinary incontinence: this procedure has an 88–90% clinical success rate.112, 113
In 1991, Vancaillie and Schuessier114 described a laparoscopic Burch procedure; since then, several other studies have confirmed the efficacy of the procedure in comparison with the standard open Burch urethropexy.115, 116 However, one prospective randomized study demonstrates that patients who had a laparoscopic Burch procedure did not have outcomes as good as those who underwent an open Burch procedure.117 Potential reasons to explain the difference could be (1) the procedure was not completed in the same fashion as the open version and (2) difficulty tensioning the sutures placed when performed laparoscopically.
In 1993, Lui118 reported performing 58 laparoscopic Burch procedures with a 94% success rate and an 8.5% complication rate. However, the patients were followed-up for only 3 months postoperatively. Das and Palmer,119 in a nonrandomized study, compared the outcomes in three groups of ten patients each who underwent a Raz vaginal needle suspension, an open colposuspension, or a laparoscopic colposuspension. The small number of patients and lack of randomization limit the study; however, outcomes in all three groups were similar. Polascik and associates120 report that 83% of patients who underwent a laparoscopic Burch colposuspension were continent after a mean follow-up of 20.8 months, and 70% of patients who underwent an open Burch colposuspension were continent after a mean follow-up of 35.6 months. The patients who underwent the laparoscopic procedure required less postoperative analgesia and had a shorter hospital stay (1–9 days versus 4.9 days); however, the laparoscopic Burch colposuspension took an average of 1.5 hours longer.
Access to the space of Retzius is frequently accomplished by inserting a Veress needle through a 10-mm incision inferior to the umbilicus to obtain a pneumoperitoneum. After insertion of a laparoscope and sleeve, three additional trocars are placed under direct visualization; one should be inserted in the midline, and the two others should be placed lateral to the rectus muscle and midway between the umbilicus and the symphysis. One of these trocars should be 10 mm. Entrance into the space of Retzius is obtained by making a transverse incision in the anterior peritoneum, 2–5 cm (1–2 inches) superior to the symphysis, which extends bilaterally to the obliterated umbilical ligaments. This method helps avoid injury to the deep inferior epigastric vessels. Methylene blue dye or indigo carmine can be placed into the bladder to alert the surgeon of bladder injury. Sharp and blunt dissection is used to develop this plane until the pubic symphysis, bladder neck, and Cooper's ligament can be identified clearly. Nezhat and coworkers121 also report using hydrodissection with the suction-irrigator probe and fluid at a pressure of 300 mmHg to develop the space of Retzius. At the end of the procedure, the peritoneum may be closed, but this is not mandated.
An extraperitoneal approach can be used in which entrance into the peritoneal cavity is avoided, an advantage for patients with known pelvic adhesions. However, this procedure may be more difficult in patients who have had previous lower abdominal surgery because scarring can make the dissection difficult. This approach also may help prevent damage to intraperitoneal organs and may decrease postoperative pain.
A midline vertical incision is made several centimeters below the umbilicus, and the rectus fascia is incised. With sharp and blunt dissection, a space is created superior to the peritoneum. This also can be performed with a balloon dissector rather than carbon dioxide gas to help develop the space of Retzius. In this procedure, the instrument is tunneled past the arcuate line until the symphysis pubis is encountered. The balloon is inflated to 1 liter to allow visualization while dissection is taking place through the balloon. When enough space has been created, other ports can be placed under direct visualization. A recent retrospective study including 47 patients showed a continence rate of 90% with follow-up of 2–15 months.122 Six per cent of the procedures had to be converted to open colposuspension because of blood loss. Neither the patient's weight nor history of previous surgery was an exclusion factor, and operating time averaged 96.4 minutes. In addition, the balloon can compress capillary lacerations until hemostasis is achieved.
Once the space of Retzius is developed, the surgeon inserts two fingers into the vagina at the urethrovesical junction. The urethrovesical junction can be identified by gentle traction on the Foley catheter, which is placed before the procedure and the bulb is filled with 20–30 mL of normal saline. Nonabsorbable suture is placed through the 10-mm port, and the suture is placed 2 cm lateral to the urethra. A second bite is performed for added stability, and the suture then is placed through the ipsilateral Cooper's ligament. An extracorporeal knot then can be placed. The first suture is placed at the proximal urethra and a second may then be placed at the urethrovesical junction for support. Tension should be similar to that performed with the traditional open technique. The same procedure is performed on the opposite side. Cystoscopy should follow to ensure that no suture material has been placed through the bladder. Excess gas is expelled, and the fascia of ports 10 mm or greater is closed in a separate layer. Skin incisions are closed with a subcuticular stitch of absorbable suture.
Several modifications of the laparoscopic Burch procedure also have been performed. Carter123 describes using an intracorporeal entrance into the space of Retzius and subsequent dissection with the Harmonic scalpel for an almost bloodless dissection of the space of Retzius. The paravaginal tissue then is sutured laparoscopically, and a 1.5-cm incision is made in the skin superior to each Cooper's ligament. Cooper's ligament is dissected for 1–1.5 cm. A Stamey needle then is introduced into the peritoneal cavity and is loaded with the suture, which has been placed through the paravaginal tissue. The needle is withdrawn back through Cooper's ligament. The knot then is tied extracorporeally, and the same procedure is performed on the opposite side. This ensures that an adequate bite of Cooper's ligament is incorporated into the urethropexy.
Ou and colleagues124 report using Prolene hernia mesh (Ethicon) and titanium staples to suspend the bladder neck. Once the appropriate dissection is performed, the surgeon's fingers are placed in the vagina to identify the urethrovesical junction. The EMS Disposable Endostapler (Ethicon) is fired twice against the surgeon's finger to ensure correct placement. The other end of this strip then is attached to Cooper's ligament with two staples. The excess mesh is trimmed, and the same procedure is performed on the opposite side. In this series, six minor postoperative complications were reported, which were self-limited. All 40 patients reported resolution or improvement of symptoms, but mean follow-up was only 6 months.
Since the introduction of the tension-free vaginal tapes (TVT) to support the midurethra, the utility of the laparoscopic Burch has been questioned. If the midurethral slings are faster, have fewer complications, and demonstrate equal efficacy to the Burch, why would a surgeon choose the colposuspension? Long-term follow-up (up to 88 months) from a group randomized to either laparoscopic Burch or tension-free vaginal tape showed comparable rates of recurrent urinary incontinence (58 vs 48%, Burch and TVT, respectively).125
Vaginal vault prolapse also can be repaired laparoscopically, with the most comprehensive repair being the sacrocolpopexy. Uterosacral vault suspension may be an option for the patient with less advanced stages of apical prolapse, but the sacrocolpopexy remains the most durable approach.126 In this procedure, a wide-pore synthetic mesh (Prolene) is introduced into the abdomen through a 10-mm suprapubic port. The material is cut to conform to the width and length of the vaginal tube (approximately 4 cm wide and 10–12 cm long). A ring forceps with a sponge or obturator is placed in the vagina for elevation. The sigmoid colon should be moved laterally to improve visualization, and the patient placed in Trendelenburg position. If necessary, the mesosigmoid can be sutured to the abdominal wall during the procedure. The iliac bifurcation, the median sacral vein, the iliac veins, and the ureter should be identified before beginning the procedure. The peritoneum overlying the sacral promontory is elevated and incised. The incision is extended inferiorly along the right pararectal area, then superiorly along the posterior vaginal wall. The bladder is dissected free from the anterior vaginal wall and the rectovaginal space dissected. Mesh is sutured to both the anterior and posterior vagina with four to six interrupted delayed-absorbable or permanent suture on each side (Gore-Tex, Prolene, PDS). Next, the mesh is attached to the sacral periosteum under appropriate tension and sutured with permanent suture or screws. The peritoneum then is closed over the mesh to minimize exposure to overlying bowel.
Nezhat and colleagues127 performed laparoscopic sacral colpopexy in 15 women. All of the procedures but one was completed laparoscopically with a mean operating time of 170 minutes. Patients were followed for 3–40 months, and all reported complete relief of symptoms with no coital difficulty. Similar results have been reported in a more recent series of 27 patients.128 Early comparative studies suggest that the laparoscopic approach is feasible, results in less blood loss and pain than its abdominal counterpart, and may demonstrate comparable efficacy.129 When offering patients without symptomatic stress urinary incontinence sacrocolpopexy as a reparative surgery for uterovaginal prolapse, a prophylactic colposuspension may decrease the risk of postoperative stress incontinence.130
Both laparoscopic Burch urethropexy and laparoscopic sacral colpopexy have proven to be beneficial and successful procedures. However, they also may prove to be more cost-effective by minimizing postoperative pain and decreasing recovery time for the patient. Further prospective randomized studies with longer follow-up periods are needed to evaluate their efficacy.
DISTAL TUBAL OCCLUSION
Tubal occlusion is reported to be responsible for 20–50% of cases of female infertility. Patients with this condition commonly are diagnosed after a HSG reveals a distal tubal blockage; blockage also may be discovered incidentally at the time of laparoscopy.
Determining when to repair a distal occlusion is based on several factors. The extent of tubal adhesions and the extent of distortion of the pelvic anatomy play an important role in ascertaining the final outcome after surgical repair. The American Society for Reproductive Medicine Classification has developed a scoring system for distal tubal occlusion to help predict postsurgical tubal function.131 Under this system, patients receive a score based on the distal ampullary diameter, tubal wall thickness, mucosal folds at the neostomy site, extent of adhesions, and type of adhesions.
Dubuisson and associates132 demonstrated that the condition of the tube during laparoscopy was as effective in predicting outcome as the distal tubal scoring system for adhesions proposed by Mage and colleagues.133 In the Mage system, patients are classified according to the amount of ovarian surface involved and the amount of the distal tube enclosed by adhesions. Also, the type of adhesion (e.g., filmy, vascular, and dense) helps to determine the final score. In this study, patients with a hydrosalpinx without mucosal folds or with severe intraluminal adhesions, resulting in a honeycomb appearance, had a prognosis equivalent to that of patients found to have severe tubal adhesions and classified as stage III or IV. Other studies also show that mucosal assessment is more important than the extent of adhesions.134, 135 The success of the repair also depends on the dilation of the ampullary region of the tube. If this region is greater than 1.5 cm, as demonstrated on HSG, the chance of subsequent pregnancy is poor.136 In the same study, biopsy was performed on the fimbria during laparoscopy, and a significantly lower number of cilia were present in patients with hydrosalpinges greater than 1.5 cm in diameter.
Ideally, the preoperative workup should yield negative results for any evidence of anovulation, male factor, or any condition that would further impair fertility. However, if a second factor is present, it must be taken into consideration when deciding whether to proceed directly to in vitro fertilization versus tubal repair.
Fimbrioplasty is dilating the area of tubal phimosis or lysing fimbrial adhesions to help restore normal anatomy. Chromotubation distends the tube and allows easier identification of fimbria. An atraumatic grasping device or a blunt probe can be used to steady the tube before fimbrioplasty. Fimbrial adhesions can be lysed with scissors or with thermal energy. Next, adhesions can be dissected bluntly by placing a grasping device in the tubal ostia. The forceps are opened within the tube and removed to lyse adhesions bluntly. Laparoscopic fimbrioplasty with the carbon dioxide laser compares favorably with outcomes after microsurgery.137, 138, 139, 140 The chance of an ectopic pregnancy, however, may be higher after laparoscopic repair.
Salpingoneoplasty, the creation of new ostium in a completely occluded tube, implies that more extensive tubal damage has taken place. Pregnancy rates after salpingoneoplasty are lower in most studies, regardless of the method used, and ectopic pregnancy rates are higher than with fimbrioplasty.141, 142 When performing this procedure, distention of the tubal obstruction is accomplished with installation of indigo carmine dye. The point of obstruction is identified as closely to the original opening as possible by looking for a dimple at the end of the tube or by looking for stellate scarring. Two atraumatic graspers then are used to grasp the tube at the 3- and 9-o'clock positions. Laparoscopic scissors or laser may be used to make the initial vertical incision from the 12- to 6-o'clock positions at the end of the tube. Once dye begins to spill from the tube, an instrument such as a suction-irrigator device can be placed in the opening. Next, vertical incisions are made at the 2-, 4-, 8-, and 10-o'clock positions on the tube. With each incision, the suction-irrigator is used as a backstop between the anterior and posterior tubal tissue. The tubal edges can be everted with a carbon dioxide laser at 5 watts of continuous power and a defocused laser beam. Laparoscopic suturing also can be done to evert the edges of the tube; however, the risk of adhesion formation is increased. Notwithstanding these surgical modalities of treating distal tubal occlusion, if the disease is significant, most would recommend proceeding to artificial reproductive technologies (ART) to improve overall fecundity.
TREATMENT OF ECTOPIC PREGNANCY
Ectopic pregnancy is becoming increasingly common in the United States: approximately 88,400 were reported in 1989.143 Of all reported pregnancies, 1.2–1.4% are ectopic,144 a finding that may result from an increase in sexually transmitted diseases and subsequent salpingitis. The increased use of ovulation induction in ART, a theoretical risk factor for ectopic pregnancy, also may contribute to this increase. Other risk factors include use of an intrauterine device or a progestin-only contraceptive, history of tubal surgery, or a previous ectopic pregnancy. However, less than 50% of patients with an ectopic pregnancy diagnosed have an identifiable risk factor.145
The development of highly sensitive assays for β-human chorionic gonadotropin (βhCG) and the use of endovaginal ultrasound have increased the likelihood of an earlier diagnosis of ectopic pregnancy and also have increased the available treatment options. Patients who are hemodynamically stable with an unruptured ectopic pregnancy, 4 cm or less, can be offered methotrexate treatment.146 Only a patient who is motivated should plan to make repeated trips to the office to evaluate the use of this medication.
Fertility outcomes in patients treated with methotrexate versus patients treated with conservative laparoscopic surgery are similar.147, 148 However, methotrexate often results in an increased time for resolution, and most information about the use of methotrexate is based on retrospective reviews.149 Laparoscopic surgery remains the gold standard for patients with unruptured ectopic pregnancies. In a retrospective study of 91 patients with ectopic pregnancy, the operating time, estimated blood loss, surgical complication rate, and hospital stay were compared in patients undergoing laparoscopy versus laparotomy for treatment.150 The operating time and estimated blood loss were similar for both groups, but the hospital stay was longer and surgical complication rate higher for the laparotomy group. In a cost analysis of operative management for ectopic pregnancy, laparoscopy with discharge on postoperative day 1 was the least expensive procedure; beginning with laparoscopy and converting this to laparotomy was the most costly approach.151
Surgical technique
Whether conservative or radical laparoscopic surgery is performed depends on the patient's desires. If the patient has an ectopic pregnancy in the ampullary portion of the tube and desires future fertility, a salpingostomy should be considered. If a conservative approach is chosen, a dilute solution of vasopressin should be injected into the antimesenteric portion of the fallopian tube. Needlepoint cautery, laser, or laparoscopic scissors are used to make a linear incision in the tube, directly over the widest portion of the ectopic pregnancy. Hydrodissection with a suction-irrigating device is useful in dissecting the ectopic tissue from the tube. With a grasping device with teeth, the tissue is extracted from the tube and placed into a laparoscopic pouch, or the tissue can be pulled directly out through a 10-mm port. Any remaining villi should be teased away from the tube. Hemostasis can be obtained with a needlepoint cautery device, or preferably with microbipolar cautery to limit thermal injury to the tube. After this procedure, the tube is left to heal with no further intervention. Values for βhCG should be obtained postoperatively until the value is less than 5 mIU/mL. Persistence of trophoblastic activity is reported to be 4–16%, and future pregnancy rates of 40–80% have been reported.152, 153, 154 Hagstrom and colleagues155 measured one βhCG level and one serum progesterone value within 24 hours before laparoscopic salpingostomy, and the βhCG value was repeated after surgery. Patients with a progesterone value of less than 11 mmol/L or a daily βhCG increase of less than 100 IU/L had a 98% chance of requiring no further therapeutic intervention.
A salpingectomy can be performed on patients who do not desire future fertility. The Harmonic scalpel can be used to coagulate and cut the area of the broad ligament inferior to the tube. Removal of the entire length of tube, including partial cornual resection, should be performed. The ipsilateral ovary is preserved when possible. After this procedure, the tube and ectopic tissue are delivered and placed in an Endobag, and then are delivered through a 10-mm port.
Dubuisson and associates156 report on 145 patients with ectopic pregnancy who underwent unilateral salpingectomy and assessment of fertility outcome. The patients were divided into two groups based on history and condition of the contralateral tube. For patients with no history of tubal surgery or infertility who had a normal contralateral tube, the pregnancy rate was 75% with a 9.6% rate of repeat ectopic pregnancy. In patients with a history of previous tubal surgery or tubal disease, the pregnancy rate was 36.6%; 18.3% experienced a repeat ectopic pregnancy. Accordingly, the authors advocate nonconservative surgery for treatment of ectopic pregnancy because the patient's fertility was minimally impaired if the contralateral mucosa appeared normal.
A review by Marana and coworkers134 supports this conclusion. Thirteen patients who had a previous salpingectomy secondary to an ectopic pregnancy underwent tubal perfusion and salpingoscopy of the remaining tube. Eight of the 13 patients with normal mucosa subsequently had an intrauterine pregnancy. Three of five patients with intra-ampullary adhesions had a repeat ectopic pregnancy. Therefore, the status of the tubal mucosa might play a role when choosing the type of surgery. The risk of leaving residual trophoblastic tissue and the risk of hemorrhagic complications after salpingectomy are less likely than with salpingostomy. However, a salpingostomy technically is less difficult.156
When the patient has rupture of a tubal segment or the pregnancy is in the isthmic portion of the tube, a segmental resection of the tube is the procedure of choice. The patient later can undergo reanastomosis of this tube.
A cornual ectopic pregnancy can be managed with laparoscopy and hysteroscopy in an appropriately selected, hemodynamically stable patient.157 If the myometrium is thick, hysteroscopic resection is advisable, and the uterus can be visualized with laparoscopy. If the pregnancy has ruptured, it may be best to perform a laparotomy.
Finally, in one study, laparoscopy was used to confirm the diagnosis of ectopic pregnancy in 33 patients.158 Patients with a decreasing βhCG who had signs of a tubal abortion or a hematosalpinx less than 2 cm, with blood loss of 50 mL or less, were managed expectantly until the βhCG level was less than 10 mIU/mL. Of the 20 patients who desired pregnancy, 16 delivered a healthy baby, one patient had a repeat ectopic pregnancy, and three were unable to conceive.
An ectopic pregnancy can be treated in many ways. The choice of approach depends largely on the patient's hemodynamic status and her desire for future fertility.
Interstitial pregnancy: new approaches
In 2000, Moon reported on a series of 24 patients with interstitial or cornual pregnancy.159 Using an Endoloop and encircling suture methods, interstitial pregnancies were managed safely, effectively, and nearly bloodlessly. Using the Endoloop method, the suture is placed around base of cornual pregnancy, an incision is made over the cornual pregnancy, an incision is made on cornua with tension kept on the Endoloop, and the conceptus then is removed. During removal, the Endoloop is tightened gradually to secure hemostasis. Cornual closure is completed simultaneously with evacuation.
LAPAROSCOPY DURING PREGNANCY
The incidence of surgical emergencies in pregnancy is 0.5–1.0%.160 An accurate diagnosis of the cause of acute abdominal pain often is difficult because of the anatomic and physiologic changes of pregnancy. Laparoscopy is useful in pregnancy because it is less invasive and the patient has less postoperative pain; it can be helpful when the diagnosis is in question. However, operative laparoscopy may increase operating time and expose the fetus to nonphysiologic levels of carbon dioxide gas. Laparoscopy during pregnancy is performed most commonly for treatment of acute appendicitis, cholecystitis, adnexal torsion, and removal of an adnexal mass.
Acute appendicitis occurs in approximately 1 in 200 pregnancies; it is the most common surgical procedure in pregnancy,161 occurring with equal frequency in each trimester.162, 163 Both fetal and maternal morbidity increase when there is perforation of the appendix. An error in diagnosis is made in about 25–40% of pregnant patients.164
Acute cholecystitis occurs in 0.03% of pregnancies. An increase in cholesterol, a decrease in bile acid secretion, and contractility of the gallbladder during pregnancy may contribute to this disease. Patients should be treated medically until the second trimester, but if symptoms do not improve, a cholecystectomy should be performed, regardless of gestational age.
Ovarian cysts have been reported to occur during the first half of pregnancy in 1 in 90 patients.165 Doppler ultrasonography can be helpful in the diagnosis of adnexal torsion, but for definitive diagnosis and treatment, operative intervention is necessary. Several successfully treated patients have been reported.166
Adnexal masses in pregnancy, requiring surgical intervention, have been reported to occur in 1 in 81–2500 pregnancies.167 Laparoscopy is especially useful when the diagnosis is uncertain. In one case report, a dermoid tumor was suspected preoperatively in a patient with a complex adnexal mass, but a leiomyomata was found at the time of surgery.168 In this procedure, the patient's anesthetic exposure, operative time, and postoperative pain were decreased because laparotomy was avoided.
Before operative intervention, the patient should receive complete fetal assessment, including ultrasound, to rule out a lethal fetal anomaly and to document cardiac activity. The optimal time for elective surgery is during the second trimester, when the fundus is less than 18 weeks' size. An association between the use of anesthetic gas and spontaneous abortion has been discovered;169 because most such cases occur during the first trimester, it is best to avoid this period when considering elective surgery. During the third trimester, laparoscopy is difficult and visualization poor because of the size of the uterus; however, a laparoscopic appendectomy has been reported as late as 25 weeks of pregnancy.170 In later gestation, consider placing a 5-mm subxiphoid port to facilitate visualization of the pelvic cavity from a higher vantage.
In addition to the usual preoperative counseling, the patient also should be informed about the increased risk of preterm labor and delivery secondary to the surgical procedure and anesthetic. The patient should be placed in left lateral tilt to avoid compression of the inferior vena cava and subsequent uteroplacental insufficiency. Rh immune globulin should be administered for an invasive procedure such as removal of an adnexal mass in an unsensitized Rh-negative mother.
An open laparoscopy with an intraumbilical incision is advisable to prevent injury to the pregnant uterus. A pneumoperitoneum is obtained with use of carbon dioxide gas. Some surgeons prefer to make a small incision in the left upper quadrant for placement of a 5-mm laparoscope. By performing this procedure, a 10-mm intraumbilical trocar can be placed under direct visualization. Others report placing the Veress needle and subsequently the trocar under ultrasonographic guidance.168
The safety of carbon dioxide to create a pneumoperitoneum in the pregnant patient remains unproven.171 Because of this concern, Lafrati and associates172 advocate using an abdominal wall lifter to establish adequate visualization during laparoscopy. They report using this technique during a cholecystectomy on a patient with a 14-week pregnancy. The surgery was completed in 1 hour, and use of the abdominal wall lifter allowed adequate exposure.
Nitrous oxide can be used for insufflation.161 However, when administered over several days at 50% concentration, it has been associated with an increase in spontaneous abortion rates, skeletal deformities, and small-for-gestational age infants.173 Additional ports can be placed under direct visualization lateral to the rectus muscle and superior to the location where this port normally is placed.
After the surgical procedure, the fetus should be placed on continuous fetal heart rate monitoring. External monitors also can be used to evaluate uterine activity. If preterm labor occurs, tocolytics should be considered for short-term treatment at any gestational age.
A surgical emergency in pregnancy is uncommon, but when it does occur, laparoscopy should be considered for treatment. This procedure can be performed safely during pregnancy, and patients have shorter recovery time and decreased postoperative pain.
COMPLICATIONS
In a review of 452 cases by Saidi and coworkers in Texas,174, 175 major complications occurred in association with operative laparoscopy in 47 patients (10.4%). Hemorrhage, ureteral injuries and fistula, and intestinal obstruction were reported. The authors conclude that the overall incidence of complications in major operative laparoscopy was 10.4%. Serious complications accounted for half of all complications. In a nationwide assessment of the incidence of laparoscopic complications in Finland,176 256 such complications were noted; 160 were noted to be minor, which included mild infection, minimal to mild hemorrhage, and failed sterilization. In addition, 96 major complications occurred, including gastrointestinal tract and vascular injuries. The total complication rate was 3.6 per 1000 procedures; the rate of major complications was 1.4 per 1000. The authors conclude that diagnostic and sterilization laparoscopy appeared to be safe; however, more complex laparoscopic procedures were associated with an unacceptably high number of serious complications requiring continuous follow-up and expertise.
Urinary tract injuries
Complications associated with specific procedures range from postoperative bleeding secondary to loosening of clips from automated stapling devices to loss of a vascular pedicle.177 Urinary tract injury in association with extensive dissection, as with LAVH, must be considered and discussed with the patient when informed consent is obtained. A total of 422 women who underwent LAVH with or without removal of the fallopian tube and ovaries were evaluated.178 Six cases of urinary bladder injury were reported. The urinary bladder injury is considered the most common complication associated with LAVH. Early detection and treatment can make a significant difference with regard to prognosis.179 Ureteral injuries often go unnoticed intraoperatively. In the patient who subsequently presents with abdominal pain in association with a mass, which can occur anytime from immediately after surgery to several days or weeks later, the possibility of urinoma should be considered. This entity requires appropriate management and often can be alleviated with cystoscopic stenting; however, resection and reanastomosis may be necessary.180
Gas embolus
Gas embolization has been reported in association with combined hysteroscopic and laparoscopic procedures. Room air embolization must be minimized by using the following procedures:181 (1) carefully monitoring of end tidal carbon dioxide; (2) ensuring that there is no excessive cervical dilation; (3) avoiding steep Trendelenburg position; (4) injecting prophylactic dilute vasopressin in the cervix; (5) decreasing exposure of the dilated cervix to room air (remove weighted vaginal speculum; keep in place last dilator used in cervix or consider occluding the vagina with a sponge); and (6) filling the hysteroscopic system with fluid by priming the system before insertion into the uterine cavity.
Incisional hernia
Port site herniations are a rare event, typically occurring less than 1% of the time. Most occur at extra-umbilical sites. The rate increases with use of larger ports, especially those larger than 10 mm. Herniations rarely occur in port defects smaller than 8 mm and so facial closure is not recommended. In a series of 19 cases, Boike and coworkers6 discuss incisional bowel herniation after laparoscopic procedures. The authors found intestinal bowel herniation to be a serious complication of laparoscopic procedures. Herniations generally occur in ports larger than 10 mm, so the fascia must be reapproximated for incisions larger than 10 mm. Patients typically present 7–10 days postoperatively with symptoms that may mimic an ileus, small bowel obstruction, or wound hematoma. Physical examination and suspicion are generally adequate for diagnosing this condition; however, computed tomography scan may be helpful. Radially expanding trocars may reduce the risk of port site herniations.
Pediatric patient
Rayman and associates182 recommend that the intraoperative examination for pediatric patients include the following, all of which will decrease complications: (1) screening for cardiopulmonary abnormalities; (2) fluid replacement to normal hydration levels only; (3) cuffed endotracheal tubes (a prerequisite for effective ventilation); (4) minute ventilation to achieve normocapnia; (5) warming of the carbon dioxide insufflation; and (6) adjustment of appropriate endpoint setting for insufflation.
Electrosurgical injuries
In 66 cases of electrosurgical injuries that were brought to litigation, the following were noted: (1) cases of injury to the bowel can present either early or late; (2) blood count indices frequently are normal; (3) emergency room personnel are poorly prepared to evaluate patients who present for care after laparoscopy; (4) resection of the area of perforation is recommended, with a specimen submitted for histologic assessment with Mallory trichrome stain (this sequence is thought to be a good risk-management approach when the injury, in truth, is unpreventable trauma); and (5) with patients who have increasing pain after a laparoscopy, either early or late, the clinician should strongly consider a bowel injury.
Hemorrhage
Hemorrhagic complications in association with major vessels, unfortunately, are a potential problem. When noted, immediate laparotomy, summoning of a vascular surgeon, and type and cross-matching of blood become imperative, because this situation is life-threatening.
The potential for undiagnosed retroperitoneal hematoma must be considered, even if the obvious source of bleeding has been identified. In addition, any patient with an unexplained significant change in vital signs should be considered for this diagnosis. Again, prompt action identifying the source of bleeding is necessary.
Injury to the inferior epigastric vessels is often accessible to bipolar coagulation. However, facial closure devices such as the Carter-Thomason may be used to ligate vessels under direct laparoscopic guidance. Another option that has been suggested, but frequently fails is the use of a Foley catheter inserted through the port, inflated, and then used to create a tamponade of the vessel. Another option is to simply suture-ligate the involved vessel from an abdominal incision.183, 184
TRAINING AND CREDENTIALING
Training
Training in operative laparoscopic procedures has become an integral part of many residency training programs. CREOG-APGO provides guidelines for educators involved with endoscopic surgical teaching. Credentialing bodies, usually hospitals, must assess additional privileges for new procedures as new and innovative endoscopic procedures evolve. The learning curve should include a didactic segment and, depending on the specific task at hand, a hands-on segment. Ideally, observation of the new technique should follow, and then direct mentoring to ensure the acquisition of necessary skills.
Postresidency surgical education should afford the surgeon a better-organized and structured approach, although there is a clear need to facilitate the educational process. Although there are no specific certifying bodies, usually proof of a didactic session with a hands-on segment is a prerequisite to the specific credentialing process. Industry continues to provide intense educational experiences with hands-on segments at specific institutions. The learning curve ideally is brought from the operating room into the laboratory with the use of virtual reality. Simulating the specific surgical procedures,185 this computer-assisted video surgery is a self-directed learning process. It should be readily capable of evaluating the learner's surgical skills and deficits, thus providing a segment of the learning curve in the laboratory setting.186 The required number of supervised procedures varies. A Japanese study demonstrates that the incidence of complications increased three-fold when only two versus ten procedures were supervised. Also, urologists who attended only an introductory training course had higher complication rates than those who had more intense subsequent training.187
An effort has been made to organize privileges into basic/diagnostic, sterilizations, and advanced, in which case more complex operative endoscopic procedures are necessary. Such complex operative endoscopic procedures would include the use of scissors, clips, electrocautery, and staplers in association with simple dissection, and more complex surgical maneuvers including suturing, extensive lysis of adhesions, LAVH, and procedures to address advanced degrees of endometriosis.188
Fellowships in minimally invasive gynecologic surgery were recently introduced in a joint venture by the American Association of Gynecologic Laparoscopists and the Society of Reproductive Surgeons of The American Society for Reproductive Medicine. These have been implemented in hopes of training skilled surgeons in advanced laparoscopy and promoting the field.
Credentialing
The process of credentialing is primarily accomplished at the local hospital level. Several guidelines for operative laparoscopic credentialing have been provided by the American College of Obstetricians and Gynecologists.
Resident physicians should keep a list of procedures that they have completed, noting whether they performed the surgery or assisted in the procedure. This would allow for documentation in preparation for credentialing.
The Society of American Gastrointestinal Endoscopic Surgeons (SAGES) has developed guidelines called Granting of Privileges for Laparoscopic General Surgery. These include attending a postgraduate course in the laparoscopic procedure and assisting an expert laparoscopic general surgeon before performing a procedure in humans. The term credentials refers to written documentation of specific training or experience. SAGES states that this might include a medical school diploma, certificate of attendance at a postgraduate course or seminar, or a statement certifying competence by preceptor. A subspecialty board certification also can be considered a type of credential. The terms credentialing and certifying have been used incorrectly to convey the granting of clinical privileges, but they refer to written confirmation of training or experience and documentation of competence, respectively. The best term is privileging, as recommended by SAGES.
Is the standard of care what we think it is?
In a commentary, Feste and Winkel presented data on an anonymous survey of 1958 practicing gynecologists attending seven national symposia. A significant number of complications were unreported. Their report states, “The standard of care is markedly different than has been suggested by the medical literature.” They suggest that skilled physicians should ensure that adequate credentialing occurs189 (Tables 6, 7, and 8).
Table 6. Incidence of laparoscopic complications
| Complication | n | % |
| Bleeding trocar site | 892 | 45.6 |
| Major vessel injury | 99 | 5.0 |
| Ureter injury | 74 | 3.8 |
| Bowel injury | 343 | 17.5 |
| Bladder injury | 172 | 8.8 |
| None | 378 | 19.3 |
Table 7. Modality responsible for injury
| Major Vessels (%) | Ureter (%) | Bowel (%) | Bladder (%) | |
| Monopolar cautery | 03.2 | 10 | 20.5 | 29.6 |
| Bipolar cautery | 01.6 | 20 | 17.9 | 11.1 |
| CO2 | 01.6 | 00 | 02.6 | 0. |
| Argon laser | 01.6 | 00 | 02.6 | 03.7 |
| YAG laser | 01.6 | 00 | 02.6 | 0. |
| Endo-GIA stapler | 06.5 | 20 | 02.6 | 0. |
| Trocar | 59.7 | 00 | 10.8 | 40.7 |
| Veress needle | 14.5 | 10 | 10.3 | 03.7 |
| Unknown | 09.7 | 40 | 10.3 | 11.1 |
Table 8. Procedure during which injury occurred
| Procedure | Range (%) |
| LAVH | 21.2–22.8 |
| Unilateral SO or BSO | 10.7–15.8 |
| Endometriosis surgery | 7.9–9.4 |
| Adhesiolysis | 13.2–16.3 |
| Myomectomy | 0–0.9 |
| Ectopic pregnancy | 3.9–5.5 |
| Neosalpingostomy | 0–0 |
| LUNA procedure | 0–1.3 |
| Parasacral neurectomy | 0–0 |
| Tubal ligation | 6.6–26.7 |
| Diagnostic laparoscopy | 18.4–30.3 |
BSO, bilateral salpingo-oophorectomy; LAVH, laparoscopic-assisted vaginal hysterectomy; LUNA, laparoscopic uterosacral nerve ablation; SO, salpingoophorectomy.
ACKNOWLEDGMENTS
We thank Dr Mazin Abdullah for data processing and graphics, and Drs Maher A. Abdallah and Abby Eblen from the University of Louisville School of Medicine for their contribution to previous editions.
REFERENCES
Palter S, Olive D: Office microlaparoscopy under local anesthesia for chronic pelvic pain. J Am Assoc Gynecol Laparosc 3: 259, 1996 |
|
DeQuattro N, Hibbert M, Buller J et al: Microlaparoscopic tubal ligation under local anesthesia. J Am Assoc Gynecol Laparosc 5: 55, 1998 |
|
Love B, McCorvey R: No scalpel, no IV, no stitch: Microendoscopic office laparoscopy under local anesthesia. J Soc Laparosc Surg 1: 227, 1997 |
|
Almeida D, Val-Gallas J: Office microlaparoscopy under local anesthesia in the diagnosis and treatment of chronic pelvic pain. J Am Assoc Gynecol Laparosc 5: 407, 1998 |
|
Kochli O: Endobag extractor to remove masses during laparoscopy. Obstet Gynecol 95: 304, 2000 |
|
Boike GM, Miller CE, Spirtos NM et al: lncisional bowel herniations after operative laparoscopy: A series of nineteen cases and review of the literature. Am J Obstet Gynecol 172: 1726, 1995 |
|
Hersehiag A: Femoral neuropathy after laparoscopy: Case report. J Reprod Med 35: 575, 1990 |
|
Kase EH, Stiles JA: The effect of various surgical positions on vital capacity. Anesthesiology 7: 29, 1946 |
|
Mittendorf R, Aronson MP, Berry RE et al: Avoiding serious infections associated with abdominal hysterectomy: A meta-analysis of antibiotic prophylaxis. Am J Obstet Gynecol 169: 1119–1124, 1993 |
|
The American College of Obstetricians and Gynecologists: Clinical Management Guidelines for Obstetrician-Gynecologists. Antibiotic prophylaxis for gynecologic procedures, No. 23, January 2001 |
|
Geerts WH, Heit JA, Clagett GP et al: Prevention of Venous Thromboembolism. Chest 119: 132–175, 2001 |
|
Catheline JM, Turner R, Gaillard JL: Thromboembolism in Laparoscopic Surgery: Risk Factors and Preventive Measures. Surg Laparosc Endosc Percut Tech 9:1 35–139, 1999 |
|
Nguyen NT, Hinojosa MW, Fayad C et al. Laparoscopic surgery is associated with a lower incidence of venous thromboembolism compared with open surgery. Ann Surg 246: 1021–7, 2007 |
|
Childers E, Harch K, Surwit A: Office laparoscopy and biopsy for evaluation of patients with intraperitoneal carcinomatosis using a new optical catheter. Gynecol Oncol 47: 337, 1992 |
|
Feste J: Use of optical catheters for diagnostic office laparoscopy. J Reprod Med 41: 307, 1996 |
|
Byron J, Markenson G, Miyazawa K: A randomized comparison of Veress needle and direct trocar insertion for laparoscopy. Surg Gynecol Obstet 177 :259, 1993 |
|
Mlyneck M, Truska A, Garay J: Laparoscopy without the use of the Veress needle: Results in a series of 1600 procedures. Mayo Clin Proc 69: 1146, 1994 |
|
Hasaniya N, Kasasa T, Shuh T et al: Direct laparoscopic entry using a sharp and dull trocar technique. Obstet Gynecol 88: 620, 1996 |
|
Perone N: Laparoscopy using a simplified open technique: A review of 585 cases. J Reprod Med 37: 921, 1992 |
|
Penfield A: How to prevent complications of open laparoscopy. J Reprod Med 30: 660, 1985 |
|
Oshinsky G, Smith A: Laparoscopic entry instrumentation: Product options and designs. Contemp Obstet Gynecol Tech 44: 1, 1993 |
|
Mettler L: Clinical experience with an optical access trocar in gynecological laparoscopy-pelviscopy. J Soc Laparosc Surg 1: 315, 1997 |
|
Rick S, Bachmann K, Gaiselmann T et al: A new insufflation needle with a special optical system for use in laparoscopic procedures. Obstet Gynecol 84: 476, 1994 |
|
Hurd W, Bude R, Delancey J et al: The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol 171: 642, 1994 |
|
Corson S, Batzer F, Gocial B: Measurement of the force necessary for laparoscopic trocar entry. J Reprod Med 34: 282, 1989 |
|
Eddie G, White S: A comparison of reusable vs. disposable laparoscopic instrument costs Aust NZ J Surg 671: 304, 1996 |
|
Janicki T: The new sensor-equipped Veress needle. J Am Assoc Gynecol Lap 1: 154, 1994 |
|
McDougall E, Figenshaw R, Clayman R et al: Laparoscopic pneumoperitoneum: Impact on body habitus. J Laparoendosc Surg 6: 385, 1994 |
|
Kabukoba J, Skillern L: Coping with extraperitoneal insufflation during laparoscopy: A new technique. Obstet Gynecol 80: 144, 1992 |
|
Nezhat C, Nezhat F, Lucian A et al: Operative Gynecologic Laparoscopy: Principles and Techniques. pp 18–23, New York, McGraw-Hill, 1995 |
|
Doppler D: Laparoscopic instrumentation, video imaging, and equipment disinfections and sterilization. Surg Clin North Am 72: 1021, 1992 |
|
Birkett D: Three-dimensional laparoscopy. J Laparo Surg 5: 327, 1995 |
|
Kadar N, Reich H, Lui C et al: Incisional hernias after major laparoscopic gynecologic procedures. Am J Obstet Gynecol 168: 1493, 1993 |
|
Yuen D: Early incisional hernia following laparoscopic surgery. Aust NZ J Obstet Gynaecol 35: 211, 1995 |
|
Patterson M, Walters D, Browder W: Postoperative bowel obstruction following laparoscopic surgery. Am Surg 59: 653, 1993 |
|
Watson J: Sacrospinous ligament colpopexy: New instrumentation applied to a standard gynecologic procedure. Obstet Gynecol 88: 883, 1996 |
|
Delia Badia C: New suturing device for laparoscopic hysterectomy. Obstet Gynecol 85: 636, 1995 |
|
Reich H, Clarke H, Sekel L: A simple method for ligating with straight and curved needles in operative laparoscopy. Obstet Gynecol 79: 143, 1992 |
|
Levine R: Instrumentation. In: Sanfilippo J, Levine R (eds): Operative Gynecologic Endoscopy. pp 34–35, New York, Springer-Verlag, 1996 |
|
Topel H: Laparoscopic suturing techniques. In: Sanfilippo J, Levine R (eds): Operative Gynecologic Endoscopy. pp 270–277, New York, Springer-Verlag, 1996 |
|
Clarke H: Laparoscopy: New instruments for suturing and ligation. Fertil Steril 23: 274, 1972 |
|
Clarke H: An improved ligator in operative laparoscopy. Obstet Gynecol 83: 299, 1994 |
|
Puttick M, Nduka C, Darzi A: Extracorporeal knot tying using an atraumatic Babcock clamp. J Laparoendosc Surg 4: 339, 1994 |
|
Bauer O, Kupker W, Felberbaum R et al: Small-diameter laparoscopy (SDL) using a microlaparoscope. J Assist Reprod Genet 13: 298, 1996 |
|
Risquez F, Pennehoaut G, McCorvey R et al: Diagnostic and operative microlaparoscopy: a preliminary multicenter reprt. Hum Reprod 12: 1645, 1997 |
|
Downing B, Wood C: Initial experience with a new microlaparoscope 2 mm in external diameter. Aust NZ J Obstet Gynaecol 35: 202, 1995 |
|
Amaral J: The experimental development of an ultrasonically activated scalpel for laparoscopic use. Surg Laparosc Endosc 4: 92, 1994 |
|
McCarus S: Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc 3: 601, 1996 |
|
Davies H: A review of robotics in surgery. Proc Inst Mech Eng 214: 129, 2000 |
|
Kavousis L, Moore R, Adams J et al: Comparison of robotic versus human laparoscopic camera control. J Urol 154: 2134, 1995 |
|
Falcone T, Goldberg J, Margossion H et al: Robotic-assisted laparoscopic microsurgical tubal anastamosis: A human pilot study. Fertil Steril 73: 1040, 2000 |
|
Kavoussi L: Telerobotic assisted laparoscopic surgery: Initial laboratory and clinical experience. J Urol 44: 15, 1994 |
|
Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomecgtomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasiv Gynecol 14: 698–705, 2007 |
|
Giorlandino C, Bilancioni E, Bagotan P et al: Antenatal ultrasonographic diagnosis and management of fetal ovarian cysts. lnt J Gynaecol Obstet 44: 27, 1994 |
|
Van Der Zee DC, van Seumeren QP, Bax KM et al: Laparoscopic approach to surgical management of ovarian cysts in the newborn. J Pediatr Surg 30: 42, 1995 |
|
Sanfilippo I, Lobe TE: Operative laparoscopy in the pediatric patient. In: Vitale G, Sanfilippo J, Perissat I (eds): Laparoscopic Surgery: An Atlas for General Surgeons. Philadelphia, JB Lippincott, 1995 |
|
Davidoff AM, Hebra A, Kerr J et al: Laparoscopic oophorectomy in children. J Laparoendosc Surg 6(S1): SI15, 1996 |
|
Schier F, Wildschmidt J: Laparoscopy in children with ill-defined abdominal pain. Surg Endosc 8: 97, 1994 |
|
Canis MA, Mage G, Pouly JL et al: Laparoscopic diagnosis of adnexal cystic masses: A 12-year experience with long-term follow-up. Obstet Gynecol 83: 707, 1994 |
|
Ulrich U, Keckstein J, Paulus W et al: Endoscopic surgery for mature teratoma of the ovary. Surg Endosc 10: 900, 1996 |
|
Fiedler EP, Guzick DS, Guido RS et al: Adhesion formation from release of dermoid contents in the peritoneal cavity and effect of copious lavage: a prospective, randomized, blinded, controlled study in a rabbit model. Fertil Steril 65: 852–859, 1996 |
|
Saizer H: Ovarian tumours. Drugs Exp Clin Res 2: 119, 1986 |
|
Wenzl R, Lehner R, Husslein P et al: Laparoscopic surgery in cases of ovarian malignancy: An Austria-wide survey. Gynecol Oncol 63: 57, 1996 |
|
Marana R, Caruana P, Muzii L et al: Operative laparoscopy for ovarian cysts: Excision vs. aspiration J Reprod Med 41: 435, 1996 |
|
Liguori G, Tolino A, Moccia G et al: Laparoscopic ovarian treatment in infertile patients with polycystic ovarian syndrome (PCOS): Endocrine changes and clinical outcome. Gynecol Endocrinol 10: 257, 1996 |
|
Gurgan T, Yaraii H, Urman B: Laparoscopic treatment of polycystic ovarian disease [review]. Hum Reprod 9: 573, 1994 |
|
Rose BI: Laparoscopic management of polycystic ovarian disease. Curr Opin Obstet Gynecol 7: 273, 1995 |
|
Kaaijk EM, Beek JF, van ter Veen F: Laparoscopic surgery of chronic hyperandrogenic anovulation [review]. Lasers Surg Med 16: 292, 1995 |
|
Farquhar, Vandekerkhove C, Lilford P: Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Library 4: 1, 2002 |
|
Cohen Z, Shinhar D, Kopemik G et al: The laparoscopic approach to uterine adnexal torsion in childhood. J Pediatr Surg 31: 1557, 1996 |
|
Merritt DF: Torsion of the uterine adnexa: A review. Adol Pediatr Gynecol 4:3, 1991 |
|
Pena A, Ufberg D, Couney N et al: Usefulness of Doppler sonography in the diagnosis of ovarian torsion. Fertil Steril 73: 1047, 2000 |
|
Averette HE, Nguyen HN: The role of prophylactic oophorectomy in cancer prevention [review]. Gynecol Oncol 55: S38, 1994 |
|
Parker WH, Broder MS, Shoupe D, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol 106: 219–26, 2005 |
|
Chi D, Curis J: Gynecologic cancer and laparoscopy. Obstet Gynecol Clin North Am 26: 201, 1999 |
|
Nezhat CR, Burrell MO, Nezhat FR et al: Laparoscopic radical hysterectomy with paraaortic and pelvic lymph node dissction. Am J Obstet Gynecol 166: 864, 1992 |
|
Spirtos NM, Schlaerth JB, Kimball RE et al: Laparoscopic radical hysterectomy (type III) with aortic and pelvic lymphadenectomy. Am J Obstet Gynecol 175: 1763, 1996 |
|
Katz E, Donesky BW: Laparoscopic tubal reanastomosis. J Reprod Med 39: 497, 1994 |
|
Dubuisson JB, Swolin K: Laparoscopic tubal anastomosis (the one-stitch technique): Preliminary results. Hum Reprod 10: 2044, 1995 |
|
Yoon TK, Sung HR, Cha S et al: Fertility outcome after laparoscopic microsurgical tubal anastomosis. Fertil Steril 67: 18, 1997 |
|
Lee CL, Lai YM, Huang HY et al: Laparoscopic rescue after tubal anastomosis failure. Hum Reprod 10: 1806, 1995 |
|
Bissonnette F, Lapensee L, Bouzeyen R: Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril 72: 549, 1999 |
|
Rodgers AK, Goldberg JM, Hammel JP, et al. Tubal anastomosis by robotic compared with outpatient minilaparotomy. Obstet Gynecol 109: 1375–80, 2007 |
|
Harris W: Uterine dehiscence following laparoscopic myomectomy. Obstet Gynecol 80: 545, 1992 |
|
Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasiv Gynecol 14: 362–4, 2007 |
|
Sizzi O, Rossetti A, Malzoni M.et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasi Gynecol 14: 453–62, 2007 |
|
Sizzi O, Rossetti A, Malzone M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasiv Gynecol 14: 453–62, 2007 |
|
Miller C: Laparoscopic myomectomy in the infertile woman. J Am Assoc Gynecol Laparosc 3: 525, 1996 |
|
Ostrzenski A: Extensive iatrogenic adenomyosis after laparoscopic myomectomy. Fertil Steril 69: 143, 1998 |
|
Henry-Suchet J, Soler A, Loffredo V: Laparoscopic treatment of tubo-ovarian abscesses. J Reprod Med 24: 579, 1984 |
|
Reich H, Shaw F: Laparoscopic treatment of tubovarian and pelvic abcess. In: Sanfilippo JS, Levine R (eds): Operative Gynecologic Endoscopy. 2nd ed. New York, Springer-Verlag, 1996 |
|
Reich H, DeCaprio J, McGlynn R: Laparoscopic hysterectomy: I. Gynecol Surg 5: 213, 1989 |
|
Nezhat F, Nezhat C, Gordon S et al: Laparoscopic vs. abdominal hysterectomy J Reprod Med 37: 247, 1992 |
|
Summitt RL Jr, Stovall TG, Lipscomb GH et al: Randomized comparison of laparoscopy-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol 80: 895, 1992 |
|
Doucette R, Scott J: Comparison of laparoscopically assisted vaginal hysterectomy with abdominal and vaginal hysterectomy. J Reprod Med 41: 1, 1996 |
|
Borstein S, Shaber R: Laparoscopically assisted vaginal hysterectomy at a health maintenance organization: Cost effectiveness and comparison with total abdominal hysterectomy. J Reprod Med 40: 435, 1995 |
|
Nezhat C, Bess O, Dahlia A et al: Hospital cost comparison between abdominal, vaginal, and laparoscopy-assisted vaginal hysterectomies. Obstet Gynecol 83: 713, 1994 |
|
Harris M, Olive D: Changing hysterectomy patterns after introduction of laparoscopically assisted vaginal hysterectomy. Am J Obstet Gynecol 171: 340, 1994 |
|
Hur M, Kim J, Moon J et al: Laparoscopically assisted vaginal hysterectomy. J Reprod Med 40: 829, 1995 |
|
Flerges R: The ligature technique for vaginal hysterectomy. Video J Obstet Gynecol 2: 3, 1989 |
|
Parker W: Preventing ureteral injury during laparoscopic assisted vaginal hysterectomy. Contemp Obstet Gynecol 41: 88, 1996 |
|
Parker W: Laparoscopic-assisted hysterectomy: Approach with caution. Contemp Obstet Gynecol 38: 19, 1993 |
|
Shen-Gunther J: Laparoscopically assisted vaginal hysterectomy: Single-surgeon technique with minimal assistance. J Reprod Med 41: 31, 1996 |
|
Sabelia V, Chang P, Eddy C: A technique for laparoscopic completion of vaginal hysterectomy. Obstet Gynecol 87: 465, 1995 |
|
Wood C, Maher P: Laparoscopic minilaparotorny hysterectomy. Aust NZ J Obstet Gynaecol 35: 204, 1995 |
|
Henley C, Wells P: A simpler approach to hysterectomy: One-suture laparoscopic hysterectomy. J Laparoendosc Surg 4: 209, 1994 |
|
Daniell J, Kurtz B, McTavish G et al: Laparoscopically assisted vaginal hysterectomy: The initial Nashville, TN experience. J Reprod Med 38: 537, 1993 |
|
Schwartz R: Complications of laparoscopic hysterectomy. Obstet Gynecol 81: 1022, 1993 |
|
Munro M, Parker W: A classification system for laparoscopic hysterectomy. Obstet Gynecol 82: 624, 1993 |
|
Winter M: Total laparoscopic hysterectomy using the harmonic scalpel. J Soc Laparos Surg 3: 185, 1999 |
|
Marshall V, Marchetti A, Krantz K: The correction of stress incontinence by simple vesicourethral suspension. Surg Gynecol Obstet 88: 509, 1948 |
|
Burch J: Urethrovaginal fixation to Cooper's ligament for correction of stress urinary incontinence, cystocele, and prolapse. Am J Obstet Gynecol 81: 281, 1961 |
|
Burch J: Cooper's ligament urethrovesical suspension for stress urinary incontinence. Am J Obstet Gynecol 100: 764, 1968 |
|
Vancaillie T, Schuessier W: Laparoscopic bladder neck suspension. J Laparoendosc Surg 1: 169, 1991 |
|
Abala D, Schuessler W, Vancaillie T: Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol 10: 222, 1992 |
|
Nezhat C, Nezhat F, Nezhat C et al: Laparoscopic retropubic cystourethropexy. J Am Assoc Gynecol Laparosc 4: 339, 1994 |
|
Burton G: A randomized comparison of laparoscopic and open colposuspension. In Proceedings of the International Continence Society, 24th Annual Meeting Neurol Urodyn 13: 497, 1994 |
|
Lui CY: Laparoscopic retropubic colposuspension (Burch procedure). J Reprod Med 38: 526, 1993 |
|
Das S, Palmer K: Laparoscopic colposuspension. J Urol 154: 1119, 1995 |
|
Polascik T, Moore R, Rosenberg M et al: Comparison of laparoscopic and open retropubic urethropexy for treatment of stress urinary incontinence. Urology 45: 647, 195 |
|
Nezhat C, Nezhat F, Seidman D et al: A new method for laparoscopic access to the space of Retzius during retropubic cystourethropexy. J Urol 155: 1916, 1996 |
|
Flax S: The gasless laparoscopic Burch bladder neck suspension: Early experience. J Urol 156: 1105, 1996 |
|
Carter J: Laparoscopic Burch procedure for stress urinary incontinence: The Carter modification. Keio J Med 45: 168, 1996 |
|
Ou C, Presthus J, Beadle E: Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg 3: 563, 1993 |
|
Jelovsek JE, Barber MD, Karram MM, et al. Randomized trial of laparoscopic Burch colposuspension versus tension-free vaginal tape: long-term follows up. BJOG 115: 219–25, 2008 |
|
Maher C, Baessler K, Glazener CM, et al. Surgical management of pelvic organ prolapse ini women. Cochrane Database Syst Rev 18: CD004014, 2007 |
|
Nezhat C, Nezhat F, Nezhat C: Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol 84: 885, 1994 |
|
Ostrzenski A: Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet 55: 147, 1996 |
|
Hsaio KC, Latchamsetty K, Govier FE, et al. Comparison of laparoscopic and abdominal sacrocolpopexy for the treatment of vaginal vault prolapse. J Endourol 21: 926–30, 2007 |
|
Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med 354: 1557–66, 2006 |
|
American Fertility Society: Classifications of adnexal adhesions, distal tubal obstructions, tubal pregnancies, mullerian anomalies of 12 intrauterine adhesions. Fertil Steril 49: 944, 1988 |
|
Dubuisson J, Chapron C, Morice P et al: Laparoscopic salpingostomy: Fertility results according to tubal mucosal appearance. Hum Reprod 9: 334, 1994 |
|
Mage G, Pouly J, de Joliniere J et al: A preoperative classification to predict the intrauterine and ectopic pregnancy rates after distal tubal microsurgery. Fertil Steril 46: 807, 1986 |
|
Marana R, dell Acqua S, Muzii L et al: Prognostic role of laparoscopic salpingoscopy of the only remaining tube after contralateral ectopic pregnancy. Fertil Steril 63: 303, 1995 |
|
Vasquez G, Willy B, Brosen I: Prospective study of tubal mucosal lesions and fertility in hydrosalpinges. Hum Reprod 10: 1075, 1995 |
|
Donnez J, Casanas-Roux F: Prognostic factors of fimbrial microsurgery. Fertil Steril 46: 200, 1986 |
|
American Society for Reproductive Medicine Guidelines for Practice: Tubal Disease. Birmingham, AL ASRM, 1993 |
|
Tulandi T, Vilos GA: A comparison between laser surgery and electrosurgery for bilateral hydrosalpinx. Fertit Steril 44: 846, 1985 |
|
Bateman B, Nunley W, Kitchen J: Surgical management of distal tubal obstruction: Are we making progress? Fertil Steril 48: 523, 1987 |
|
Daniell I, Herbert C: Laparoseopic salpingostomy utilizing the CO2 laser. Fertil Steril 41: 558, 1994 |
|
Dubuisson J, de Joliniere J, Aubriot F et al: Terminal tuboplasties by laparoscopy: 65 consecutive cases. Fertil Steril 54: 401, 1990 |
|
Dlugi A, Reddy S, Saleh W et al: Pregnancy rates after operative endoscopic treatment of total (neosalpingostomy) or near total (salpingostomy) distal tubal occlusion. Fertil Steril 62: 913, 1994 |
|
Goldner T, Lauson H, Xia Z et al: Surveillance for ectopic pregnancy: United States, 1970–1989. MMWR Morb Mortal Wkly Rep 42: 73, 1993 |
|
Coste J, Job-Spira N, Aublet-Cuvelier B et al: Incidence of ectopic pregnancy: First results of a population-based registery in France. Hum Reprod 9:7 42, 1994 |
|
Emerson D, McCord M: Clinician's approach to ectopic pregnancy. Clin Obstet Gynecol 39: 199, 1996 |
|
Carson S, Buster J: Current concepts: Ectopic pregnancy. N Engl J Med 329: 1174, 1993 |
|
Stovall T, Ling F: Single-dose methotrexate: An expanded clinical trial. Am J Obstet Gynecol 168: 1759, 1993 |
|
Glock J, Johnson J, Brumsted J: Efficacy and safety of single-dose systemic methotrexate in the treatment of ectopic pregnancy. Fertil Steril 62: 716, 1994 |
|
Grainger D, Seifer D: Laparoscopic management of ectopic pregnancy. Curr Opin Obstet Gynecol 7: 277, 1995 |
|
Vu K, Gehbach D, Rosa C: Operative laparoscopy for the treatment of ectopic pregnancy in a residency program. J Reprod Med 41: 602, 1996 |
|
Foulk R, Steiger R: Operative management of ectopic pregnancy: A cost analysis. Am J Obstet Gynecol 175: 90, 1996 |
|
DeCherney A, Diamond M: Laparoscopic salpingostomy for ectopic pregnancy. Obstet Gynecol 70: 948, 1987 |
|
Maymon R, Shulman A, Halperin R et al: Ectopic pregnancy and laparoscopy: Review of 1197 patients treated by salpingectomy or salpingotomy. Eur J Obstet Gynecol Reprod Biol 62: 61, 1995 |
|
Zilber V, Pansky M, Bukovsky I et al: Laparoscopic salpingostomy vs. laparoscopic local methotrexate injection in the management of unraptured ectopic gestation Am J Obstet Gynecol 175: 600, 1996 |
|
Hagstrom H, Hahlen M, Bennegard-Eben B et al: Prediction of persistent ectopic pregnancy after laparoscopic salpingostomy. Obstet Gynecol 84: 798, 1994 |
|
Dubuisson J, Morice P, Chapron C et al: Salpingectomy: The laparoscopic surgical choice for ectopic pregnancy. Hum Reprod 11: 1199, 1996 |
|
Nezhat C, Nezhat F, Luciano A et al (eds): Ectopic pregnancy. Operative Gynecologic Laparoscopy: Principles and Techniques. pp 107–119, New York, McGraw-Hill, 1995 |
|
Zohav E, Gemer O, Segal S: Reproductive outcome after expectant management of ectopic pregnancy. Eur J Obstet Gynaecol Reprod Biol 66: 1, 1996 |
|
Moon H: New simple endoscopic operations for interstitial pregnancies. Am J Obstet Gynecol 182: 114, 2000 |
|
Barber H, Graber E: Surgical Diseases in Pregnancy. Philadelphia, WB Saunders, 1974 |
|
Pictrantoni M, Sanfilippo J: Endoscopic surgical procedures during pregnancy. In: Sanfilippo JS, Levine RL (eds): Operative Gynecologic Endoscopy. pp 241–253, 2nd ed. New York, Springer-Verlag, 1996 |
|
Brant H: Acute appendicitis in pregnancy. Obstet Gynecol 29: 130, 1967 |
|
Black WP: Acute appendicitis in pregnancy. Br Med J 1: 1938, 1960 |
|
Aufses A: Biliary tract disease. In: Ravensky J, Guttmacher A (eds): Pregnancy. pp 751–753, 2nd ed. Baltimore, Williams & Wilkins, 1965 |
|
Hogston P, Lilford R: Ultrasound study of ovarian cysts in pregnancy: Prevalence and significance. Br J Obstet Gynaecol 93: 625, 1986 |
|
Levy T, Dicker D, Shalev J et al: Laparoscopic unwinding of hyperstimulated ischemic ovaries during the second trimester of pregnancy. Hum Reprod 10: 1478, 1995 |
|
Tawa K: Ovarian tumors in pregnancy. J Obstet Gynecol 90: 511, 1964 |
|
Guerrieri J, Thomas K: Open laparoscopy for an adnexal mass in pregnancy: A case report. J Reprod Med 39: 129, 1994 |
|
Knill-Jones R, Newman B, Spence A: Anesthetic practice and pregnancy. Lancet ii: 807, 1975 |
|
Schreiber J: Laparoscopic appendectomy in pregnancy. Surg Endosc 4: 100, 1990 |
|
Chandra M, Shapiro S, Gordon L: Laparoscopic cholecystectomy in the first trimester of pregnancy. Surg Laparosc 4: 68, 1994 |
|
Lafrati M, Yarnell R, Schwaitzberg S: Gasless laparoscopic cholecystectomy in pregnancy. J Laparoendosc Surg 5: 127, 1995 |
|
Pederson H, Finster M: Anesthetic risk in the pregnant surgical patient. Anesthesiology 51: 439, 1979 |
|
Hulka JF, Peterson HB, Phillips JM et al: Operative laparoscopy: American Association of Gynecologic Laparoscopists 1991 Membership Survey. J Reprod Med 38: 569, 1993 |
|
Saidi MH, Vancaillie TG, White AJ et al: Complications of major operative laparoscopy: A review of 452 cases. J Reprod Med 41: 471, 1996 |
|
Harkki-Siren P, Kurki T: A nationwide analysis of laparoscopic complications. Obstet Gynecol 89: 108, 1997 |
|
Wong Sm, Levine AV, Jacobs AJ et al: Intraabdominal bleeding following laparoscopic adnexal surgery. J Reprod Med 41: 294, 1996 |
|
Lee CL, Lai YM, Soong YK: Management of urinary bladder injuries in laparoscopic-assisted vaginal hysterectomy. Acta Obstet Gynecol Scand 75: 174, 1996 |
|
Paulson JD: Laparoscopically assisted vaginal hysterectomy. J Reprod Med 41: 623, 1996 |
|
Saidi MH, Sadler RK, Vancaillie TG et al: Diagnosis and management of serious urinary complications after major operative laparoseopy. Obstet Gynecol 87: 272, 1996 |
|
Corson SL, Brooks PG, Soderstrom RM: Gynecologic endoscopic gas embolism. Fertil Steril 65: 529, 1996 |
|
Rayman R, Girotti M, Armstrona K et al: Assessing the safety of pediatric laparoscopic surgery. Surg Laparosc Endosc 5: 437, 1995 |
|
Soderstrom RM: Electrosurgical injuries during laparoscopy: Prevention and management. Curr Opin Obstet Gynecol 6: 248, 1994 |
|
Awwad JT, Isaacson K: The harmonic scalpel: An intraoperative complication. Obstet Gynecol 88: 718, 1996 |
|
Beckman CR, Lipscomb GH, Lina FW et al: Computer assisted video evaluation of surgical skills. Obstet Gynecol 85: 1039, 1995 |
|
Yamashita Y, Kurohiji T, Kakegawa T: Evaluation of two training programs for laparoscopic cholecystectorny. World J Surg 18: 279, 1994 |
|
See WA, Cooper CS, Fisher RJ: Predictors of laparoscopic complications after formal training in laparoscopic surgery. JAMA 270: 2689, 1993 |
|
Dent TL: Training and privileging for new procedures. Surg Clin North Am 76: 615, 1996 |
|
Feste J, Winkel C: Is the standard of care what we think it is? J Soc Laparosc Surg 3: 331, 1999 |